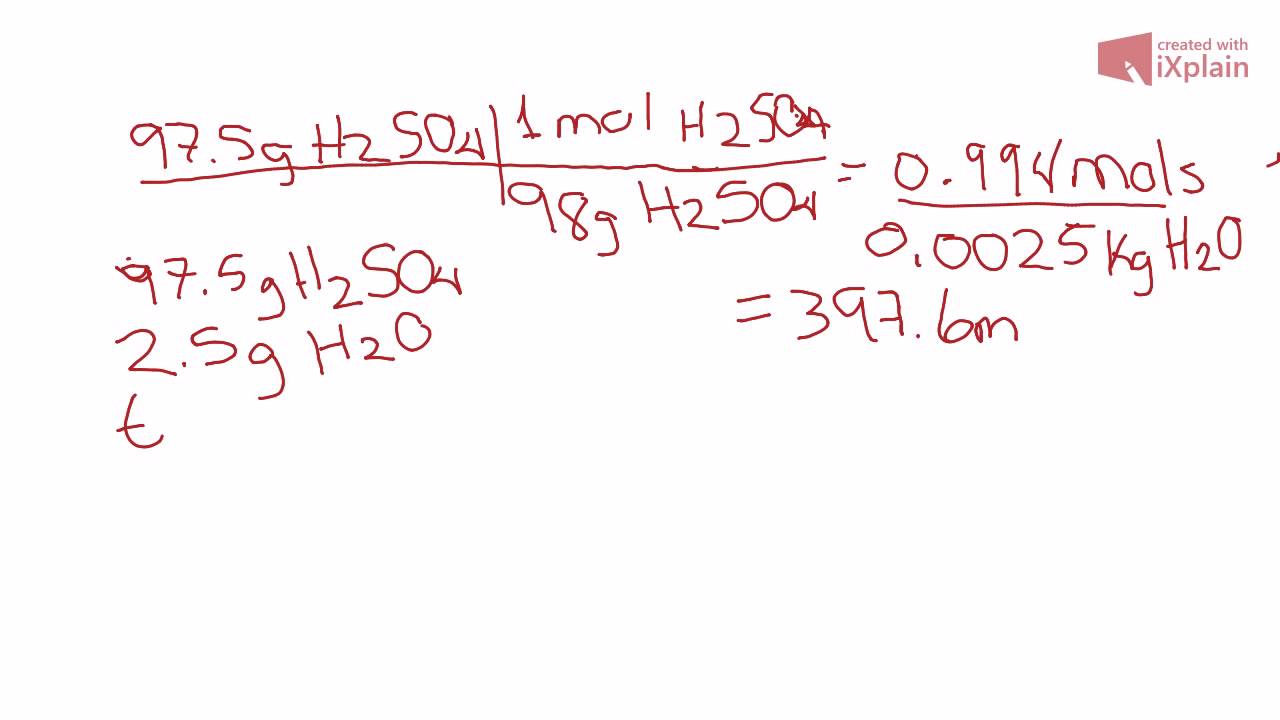Molarity of a sample of dilute sulphuric acid is 0 5 M and density is 1 02 g cm-3 - Chemistry - Some Basic Concepts of Chemistry - 13942229 | Meritnation.com
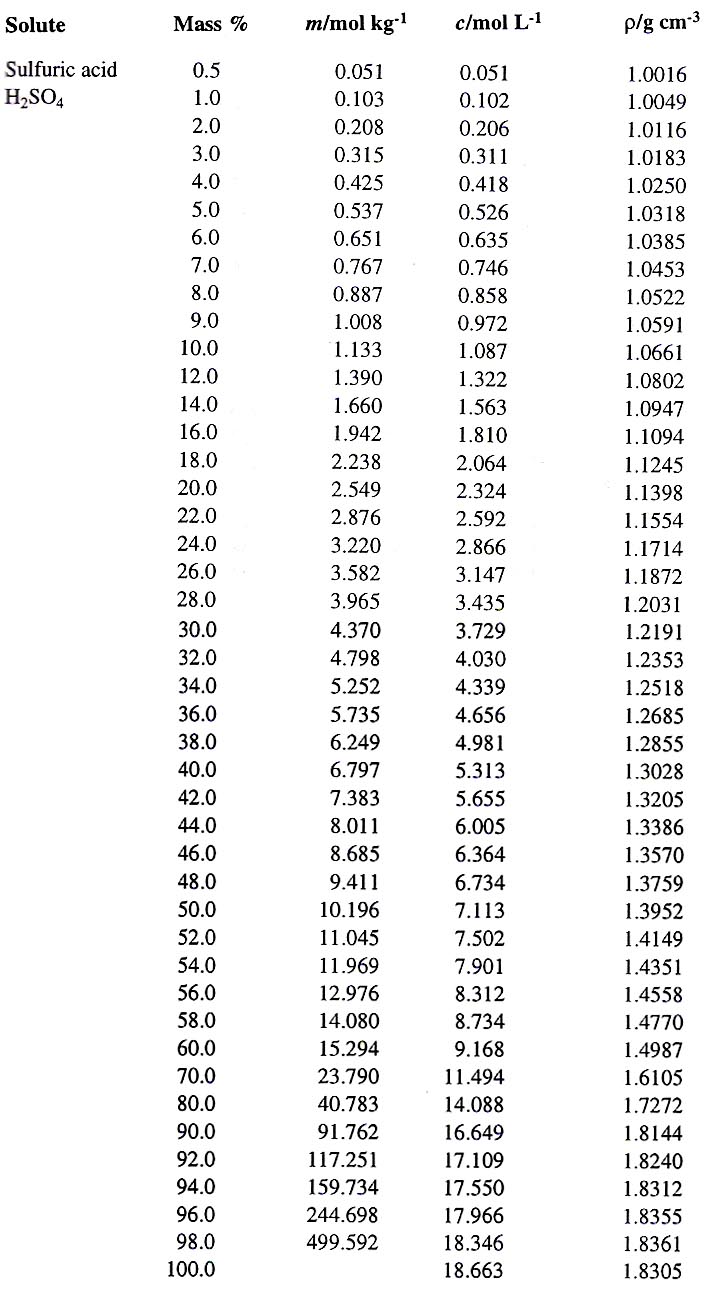
![What is the molarity of H2SO4 solution that has a density of 1.84 g/cc and contains 98% mass of H2SO4 ? [Given: the atomic mass of S = 32] What is the molarity of H2SO4 solution that has a density of 1.84 g/cc and contains 98% mass of H2SO4 ? [Given: the atomic mass of S = 32]](https://haygot.s3.amazonaws.com/questions/1308069_771116_ans_de8785a7f08b42caa8f74797b87a094b.jpg)
What is the molarity of H2SO4 solution that has a density of 1.84 g/cc and contains 98% mass of H2SO4 ? [Given: the atomic mass of S = 32]

Question Video: Determining the Concentration of Sulfuric Acid Via Titration with Sodium Carbonate | Nagwa

What is the molarity of concentrated sulfuric acid if it is 96% by mass H2so4 and has a density of 1.84g/mL? - Quora

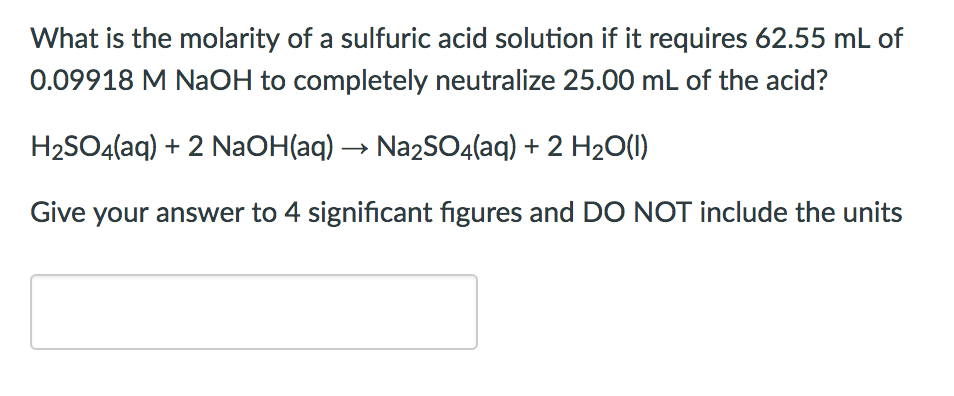
Question Video: Calculating the Volume of Sulfuric Acid That Completely Neutralizes a Given Volume and Concentration of Sodium Hydroxide | Nagwa
Chem201, Winter 2006 Name Answer key______________ Midterm N1 01/26/06 SID___________________________ 1. A solution is prepared

Ricca R8215000-500A Sulfuric Acid 500 mL, Poly Natural, 0.04 Normal,0.02 Molarity New Laboratory Setup Savings - up to 40%, Magnetic Stirrer, Vortex Mixer, Sample Prep, Centrifuge