![SOLVED: [ ] pKa of weak acids at 25*C Name Formula acetic acid CH3COzH benzoic acid C6HsCOzH butanoic acid CzHzCOzH 4-chlorobutanoic acid CzHsCICOzH crotonic acid CzHsCOzH oxalic acid HzC204 phosphoric acid HaPOa SOLVED: [ ] pKa of weak acids at 25*C Name Formula acetic acid CH3COzH benzoic acid C6HsCOzH butanoic acid CzHzCOzH 4-chlorobutanoic acid CzHsCICOzH crotonic acid CzHsCOzH oxalic acid HzC204 phosphoric acid HaPOa](https://cdn.numerade.com/ask_images/346c7f228e504822a839e28cc57b4b36.jpg)
SOLVED: [ ] pKa of weak acids at 25*C Name Formula acetic acid CH3COzH benzoic acid C6HsCOzH butanoic acid CzHzCOzH 4-chlorobutanoic acid CzHsCICOzH crotonic acid CzHsCOzH oxalic acid HzC204 phosphoric acid HaPOa
A hydrogen electrode placed in a buffer solution of CH3COOH and acetic acid in the ratio's x:y and y:x has electrode potential values E1 and E2 volts respectively at 25. the pKa

pKa value of acetic acid is 4.75. If the buffer solutions contains 0.125 M acetic acid and 0,25 M sodium acetate the pH of buffer solution is :
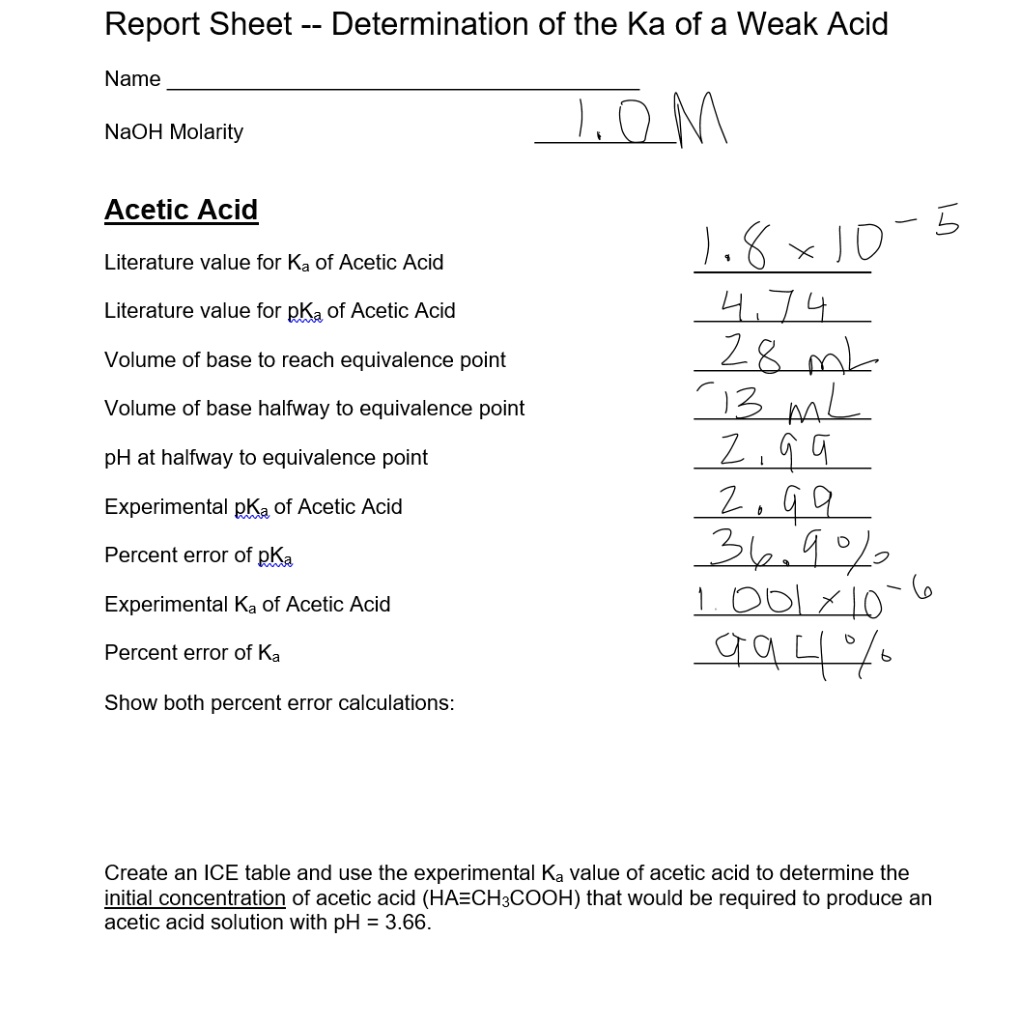
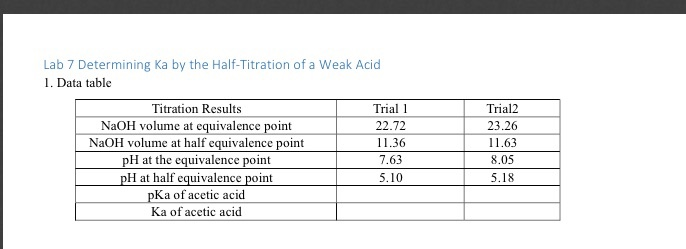
SOLVED: Report Sheet Determination of the Ka of a Weak Acid Name NaOH Molarity IM Acetic Acid Literature value for Ka of Acetic Acid Literature value for pKa of Acetic Acid LkxD -
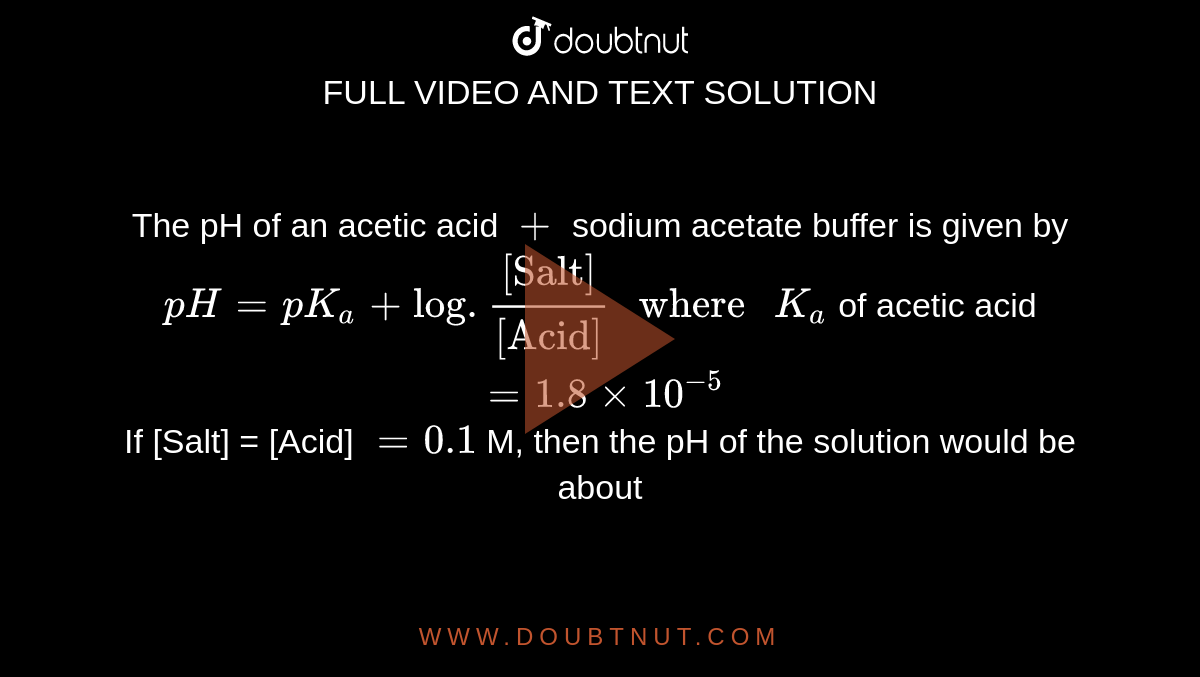
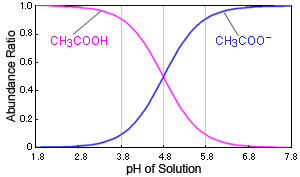
The pK(a) value of acetic acid is 4.7447 at 25^(@)C. How would you obtain a buffer of acetic acid and sodium acetate with pH = 4?
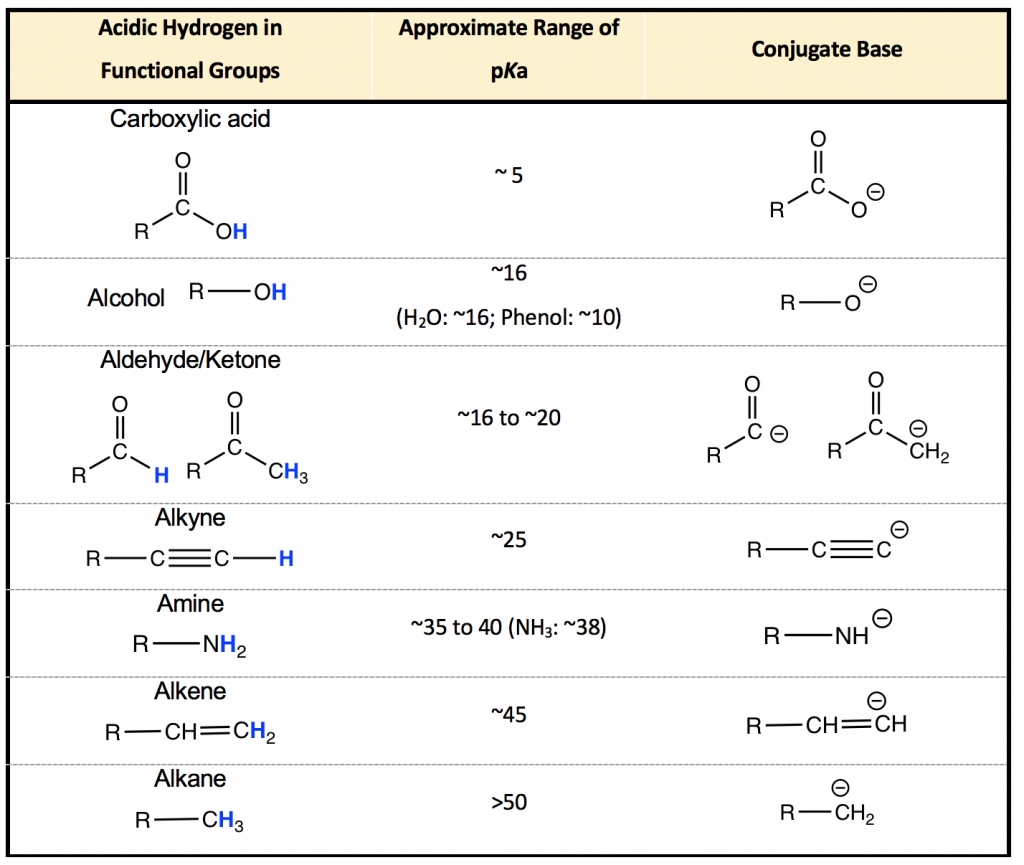
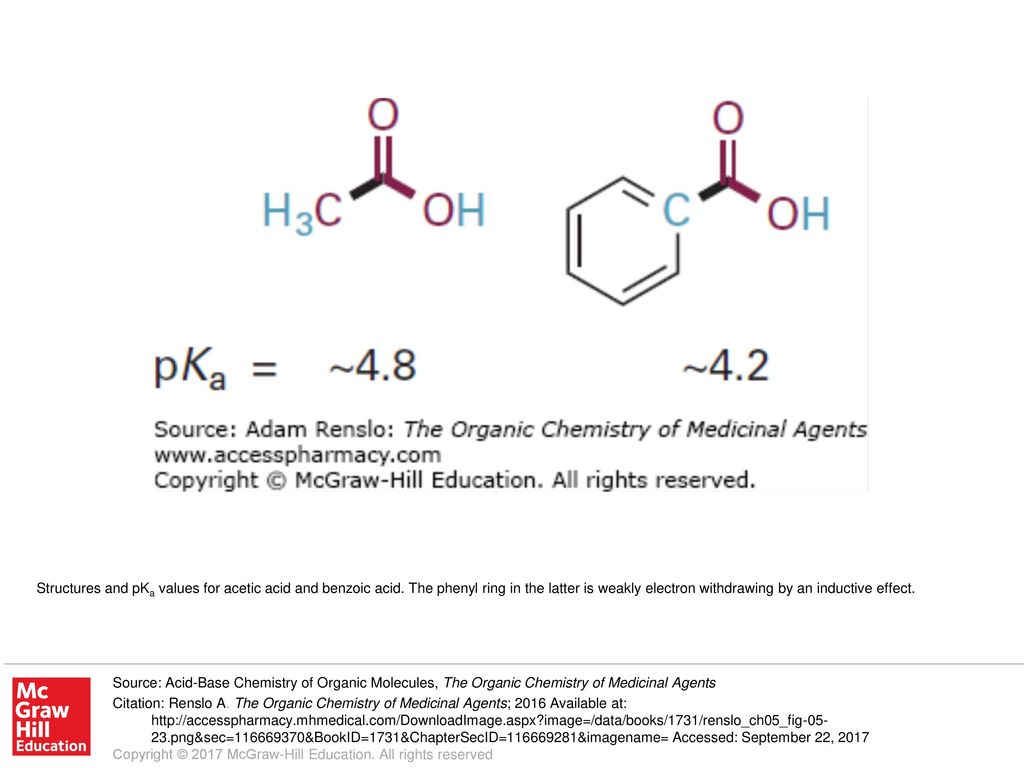
3.3 pKa of Organic Acids and Application of pKa to Predict Acid-Base Reaction Outcome – Organic Chemistry I

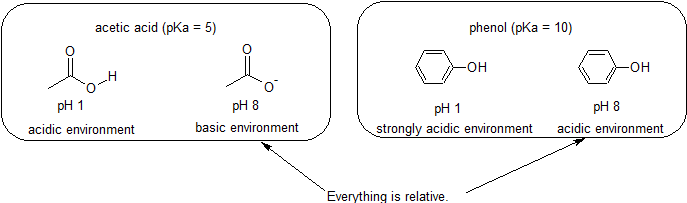
The pKa of acetic acid and pKb of ammonium hydroxide are 4.76 and 4.75 respectively......... - YouTube
The pH of an acetic acid solution is 3.26. What is the concentration of acetic acid and what is the percent of acid that's ionized? - Quora

OneClass: What concentrations of acetic acid (pKa = 4.76) and acetate would be required to prepare a ...
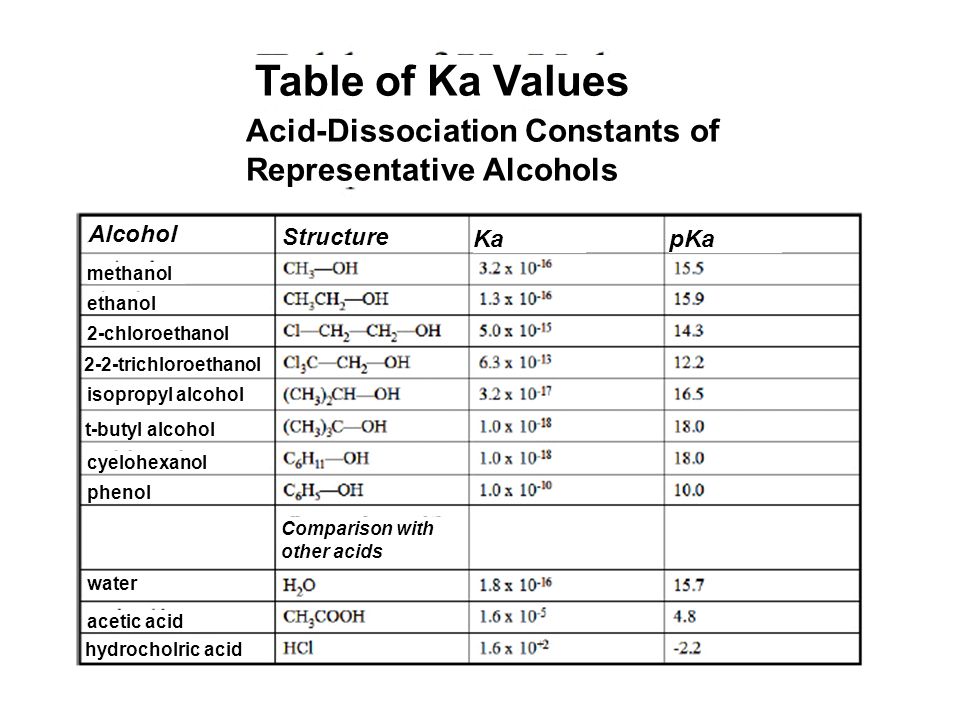
Table of Ka Values Acid-Dissociation Constants of Representative Alcohols Alcohol Structure Ka pKa methanol ethanol 2-chloroethanol 2-2-trichloroethanol. - ppt video online download

Experimental pKa values and structures of the conformers of acetic,... | Download Scientific Diagram
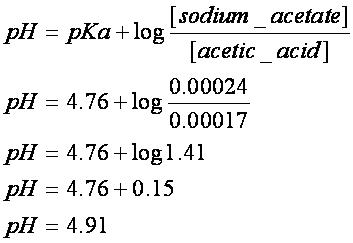
pH calculations and more in fundamentals of pharmaceutics. : Calculate pH of 100 ml buffer solution containing 0.1 g acetic acid and 0.2 g sodium actetate.




:max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)