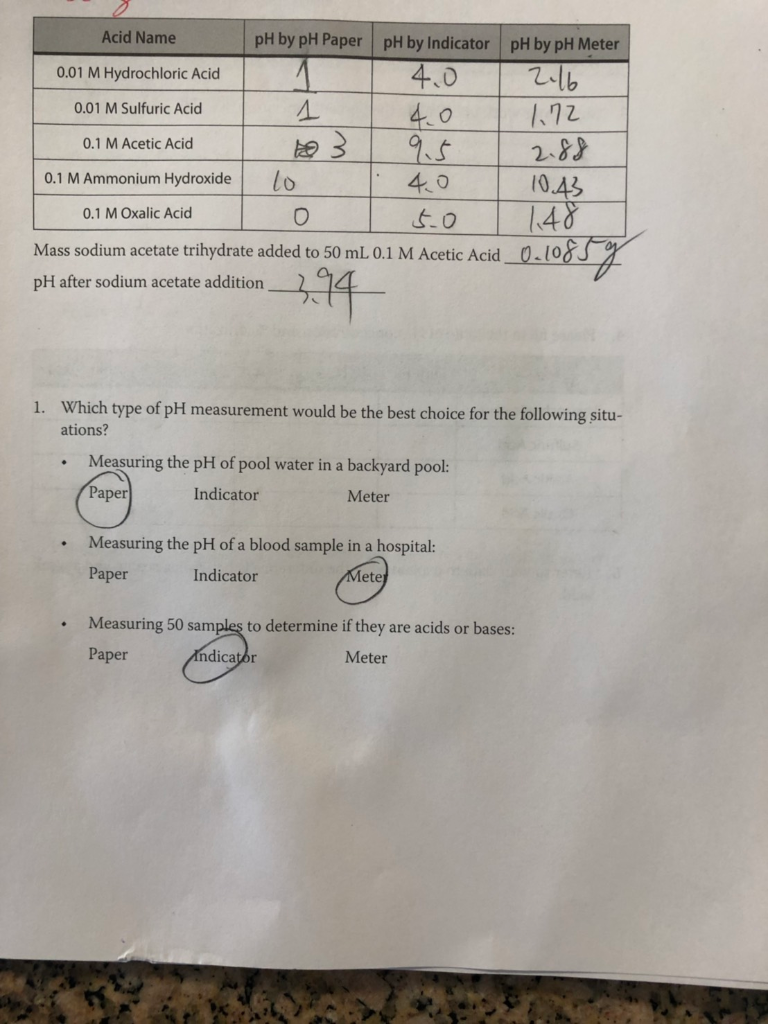
01M acetic acid solution is titrated against 0.1M NaOH solution. What would be the difference in pH between 1/4 and 3/4 stages of neutralisation of acid ?
![The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ] The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/QXVmVDZfQ29GV1k=/sd/)
The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]
Calculate the pH of 0.1M CH3COOH solution. Dissociation constant of acetic acid is 1.8 x 10^-5 - Sarthaks eConnect | Largest Online Education Community
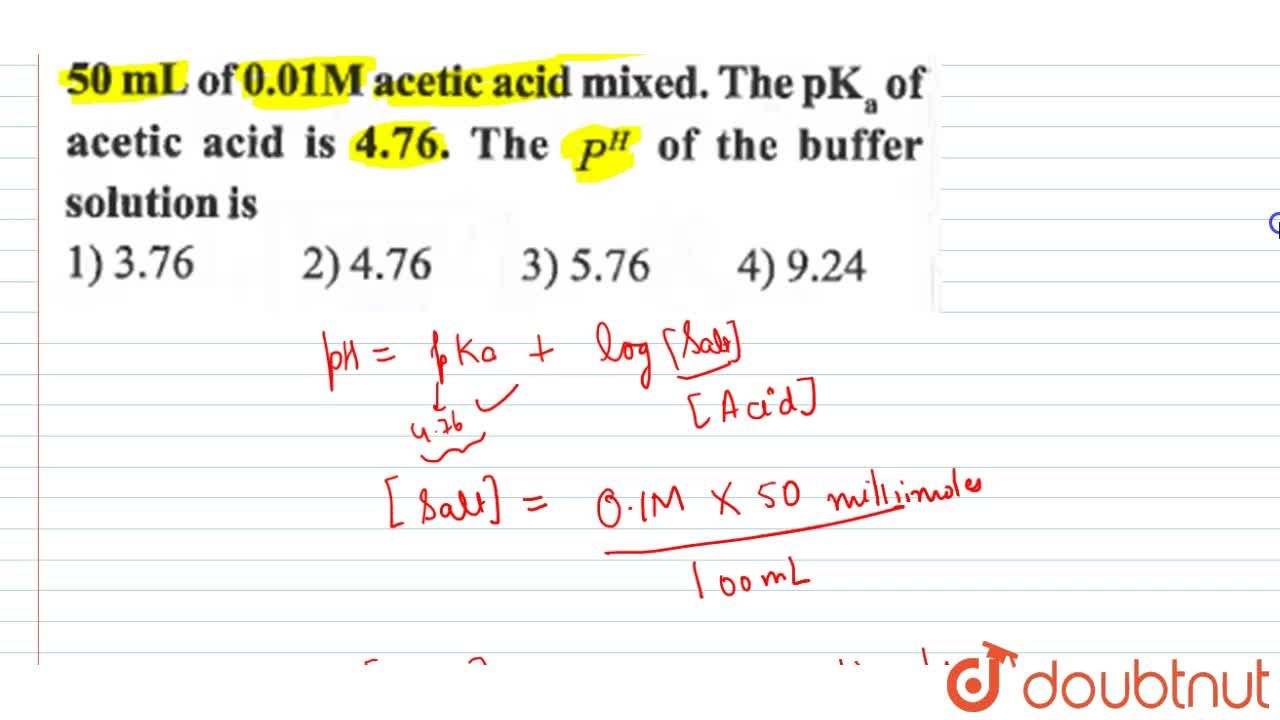
50 mL of 0.1 M solution of sodium acetate and 50 mL of 0.01 M acetic acid mixed. The pK(a) of acetic acid is 4.76. The P^(H) of the buffer solution is

Calculate ph of 0.1m solution of acetic acid if degree of dossocuation of the acid us 0.0132 - Brainly.in
![The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ] The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]](https://haygot.s3.amazonaws.com/questions/1938773_1231298_ans_7f19b0e0d221405fbf38b0df680608aa.jpg)
The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]

To a solution of 20 mL of 0.1 M acetic acid, a solution of 0.1 M NaOH is added from burette. If 'r' - YouTube

Calcualte the pH at the equivalence point when a solution of 0.1M acetic is titrated with a solution of 0.1M NaOH.(K(a)for acid = 1.9xx10^(-5)).
![The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ] The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]](https://haygot.s3.amazonaws.com/questions/1941080_1288163_ans_f3290aa7aaae4ffbbe9ff1c9f5aca233.jpg)
The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]

when 10 ml of 0 1 M acetic acid ( pka = 50) titrated against 10 ml of 01 - Chemistry - Equilibrium - 10271133 | Meritnation.com
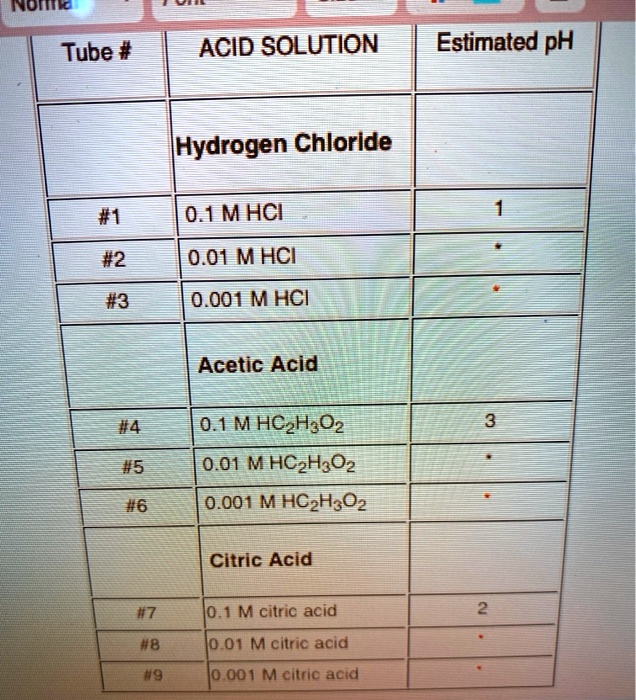
SOLVED: Inomo Tube # ACID SOLUTION Estimated pH [Hydrogen Chloride #1 0.1MHCI #2 #3 0.01 MHCI 0.001 MHCI Acetic Acid #4 0.1MHCzH;Oz 0.01 M HCzH3Oz 0.001 M HCzH3Oz #5 #6 Citric Acid