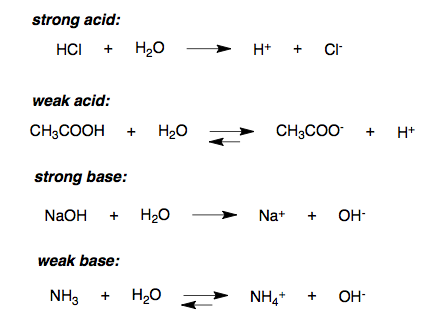
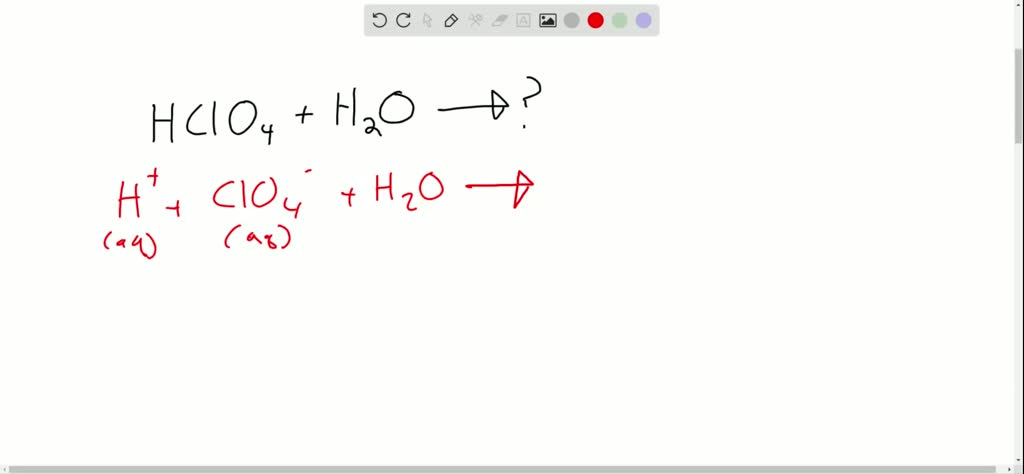
Ionization of Acid And Bases - Arrhenius concept of Acid And Base Ionisation, Explanation, Determination ionisation constant of Acid base, Examples And FAQS

Find the H_3O^+ concentration of a 0.280 M hypochlorous acid solution (whose acid dissociation constant is Ka=2.9 x 10^-8). | Homework.Study.com

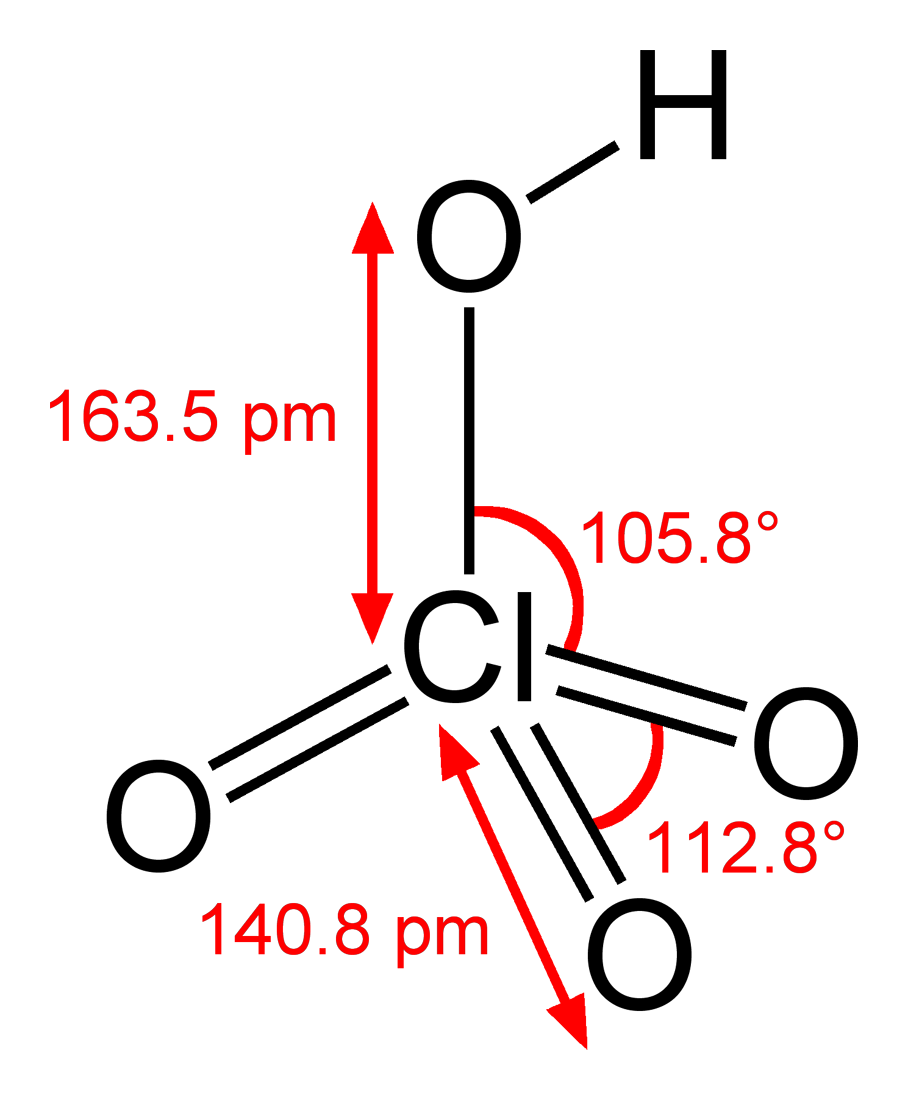
Dissociation Constants of Perchloric and Sulfuric Acids in Aqueous Solution | The Journal of Physical Chemistry B
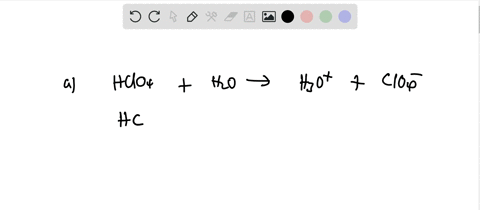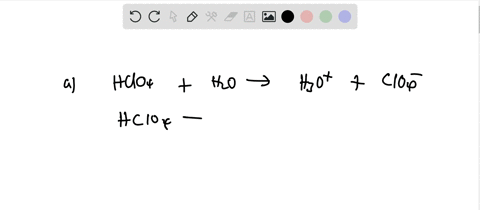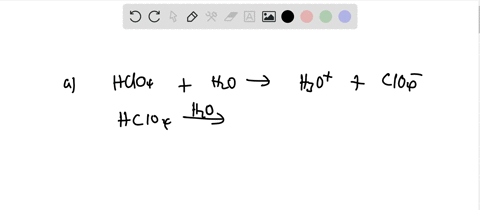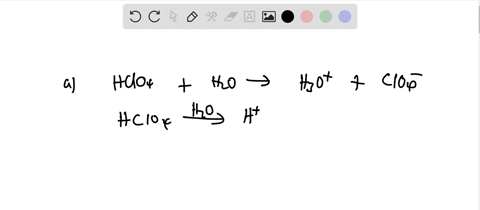
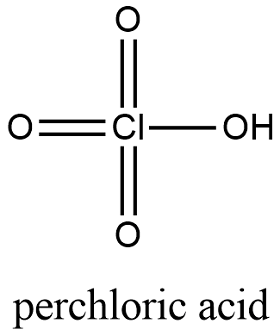
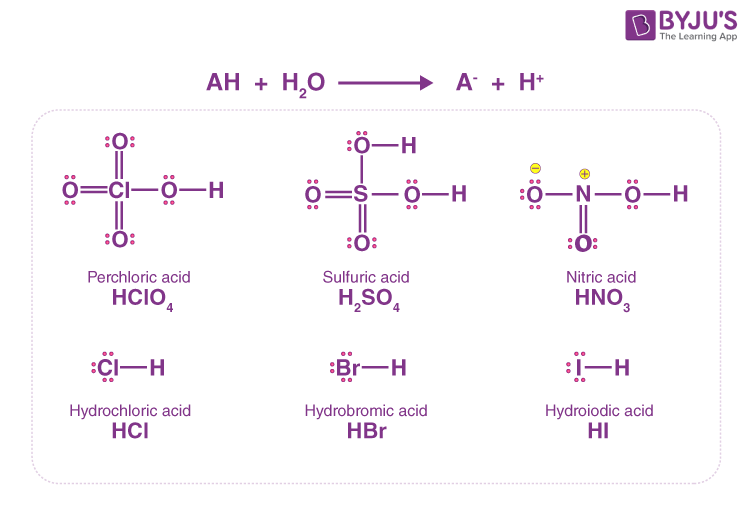
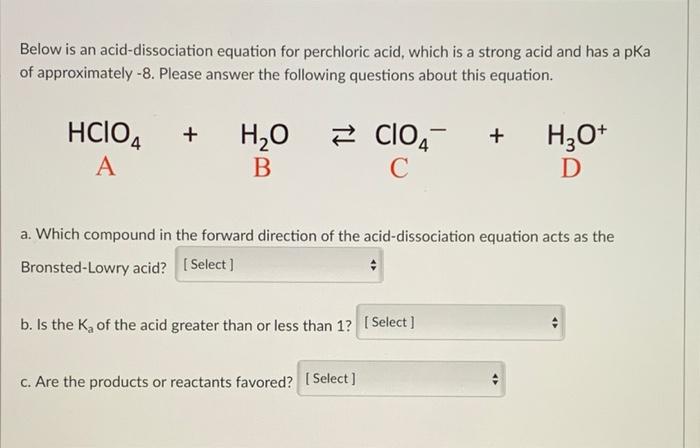
SOLVED: Perchloric acid, HClO4, is a strong acid. (a) Write the chemical equation for the reaction between perchloric acid and water. (b) List all species present in an aqueous solution of perchloric

Strong Acids Ions are present in an aqueous solution of an acid, because these ions result from the dissociation of the acid. An acid that dissociates. - ppt download