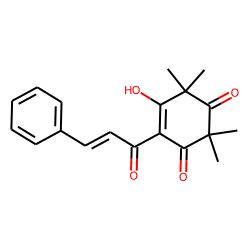
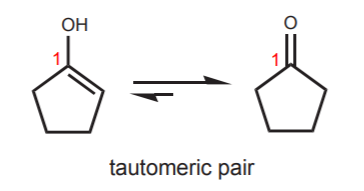
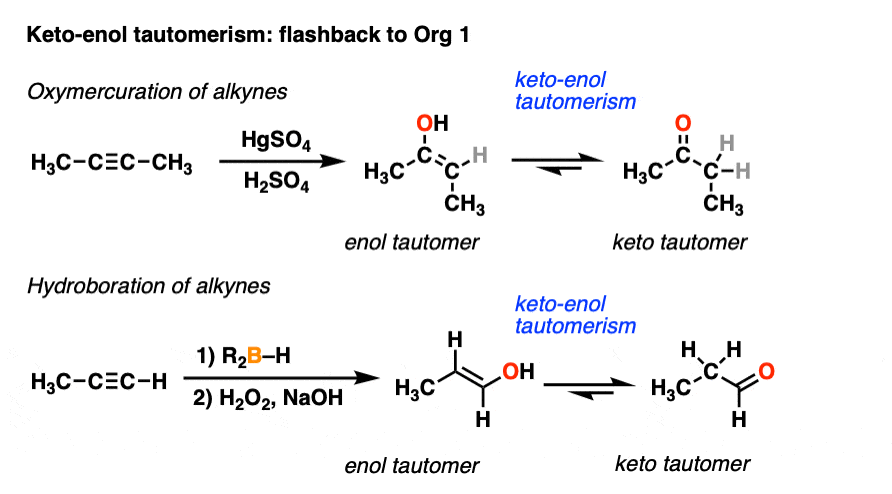
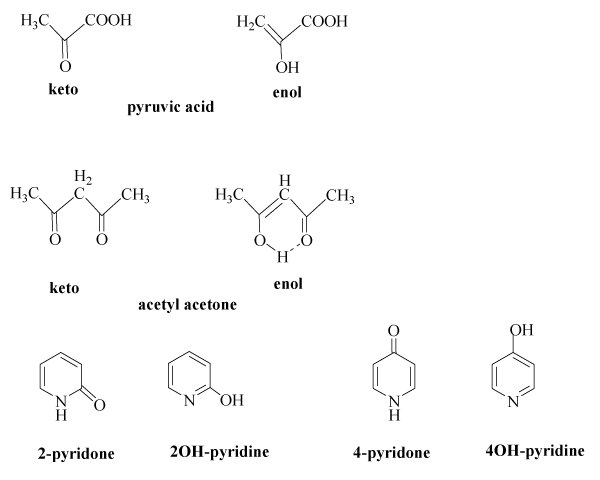
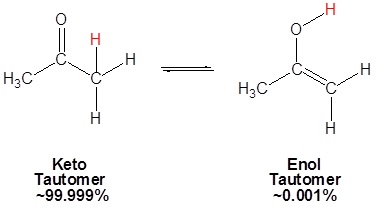
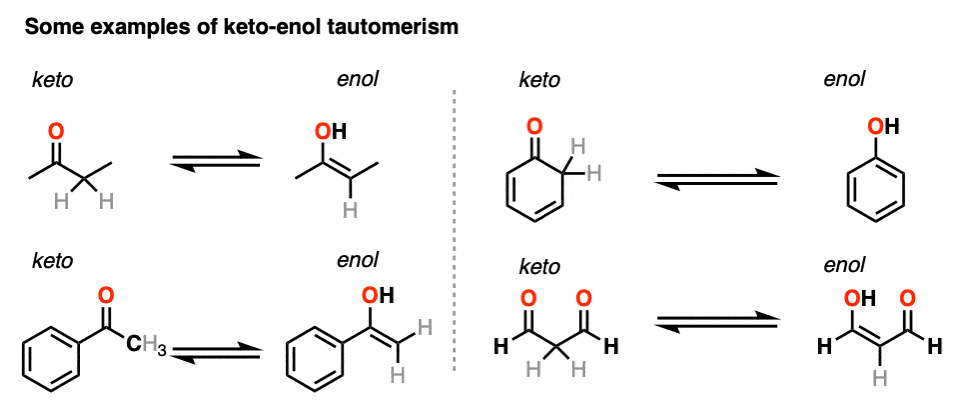
Keto-enol tautomerism of a peptide bond (A) followed by a redox process... | Download Scientific Diagram

DFT Study To Explore the Importance of Ring Size and Effect of Solvents on the Keto–Enol Tautomerization Process of α- and β-Cyclodiones | ACS Omega

PDF) An exploratory study on the oxo-enol tautomerization of selected dioxopiperazines and their sulphur-containing analogues | A. Perczel - Academia.edu

I2-Catalyzed Regioselective Oxo- and Hydroxy-acyloxylation of Alkenes and Enol Ethers: A Facile Access to α-Acyloxyketones, Esters, and Diol Derivatives | Organic Letters

Reversible Shifting of a Chemical Equilibrium by Light: The Case of Keto– Enol Tautomerism of a β-Ketoester | Organic Letters
The 2-oxo-3,3,5,5-tetramethylcyclopentanecarboxylic acid keto–enol system in aqueous solution — Generation of the enol by fl
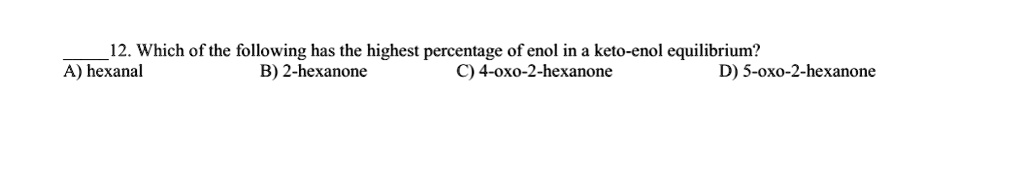
SOLVED: 12.Which of the following has the highest percentage of enol in a keto-enol equilibrium? A)hexanal B)2-hexanone C) 4-oxo-2-hexanone D) 5-oxo -2-hexanone