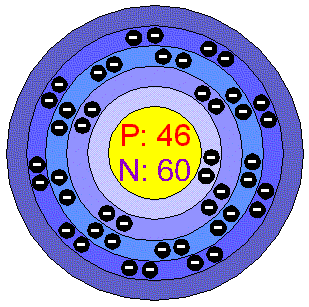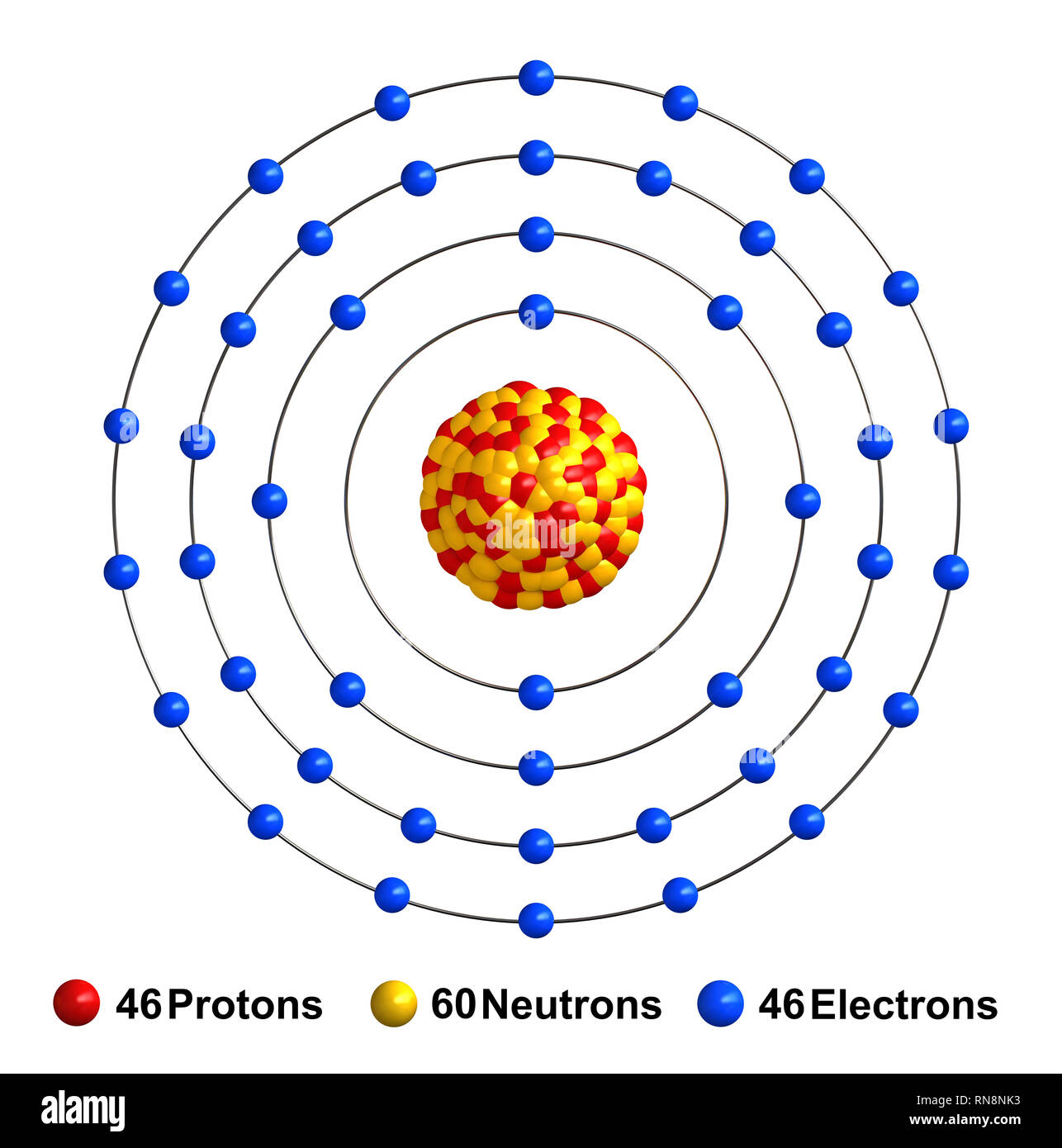
3d render of atom structure of palladium isolated over white background Protons are represented as red spheres, neutron as yellow spheres, electrons a Stock Photo - Alamy
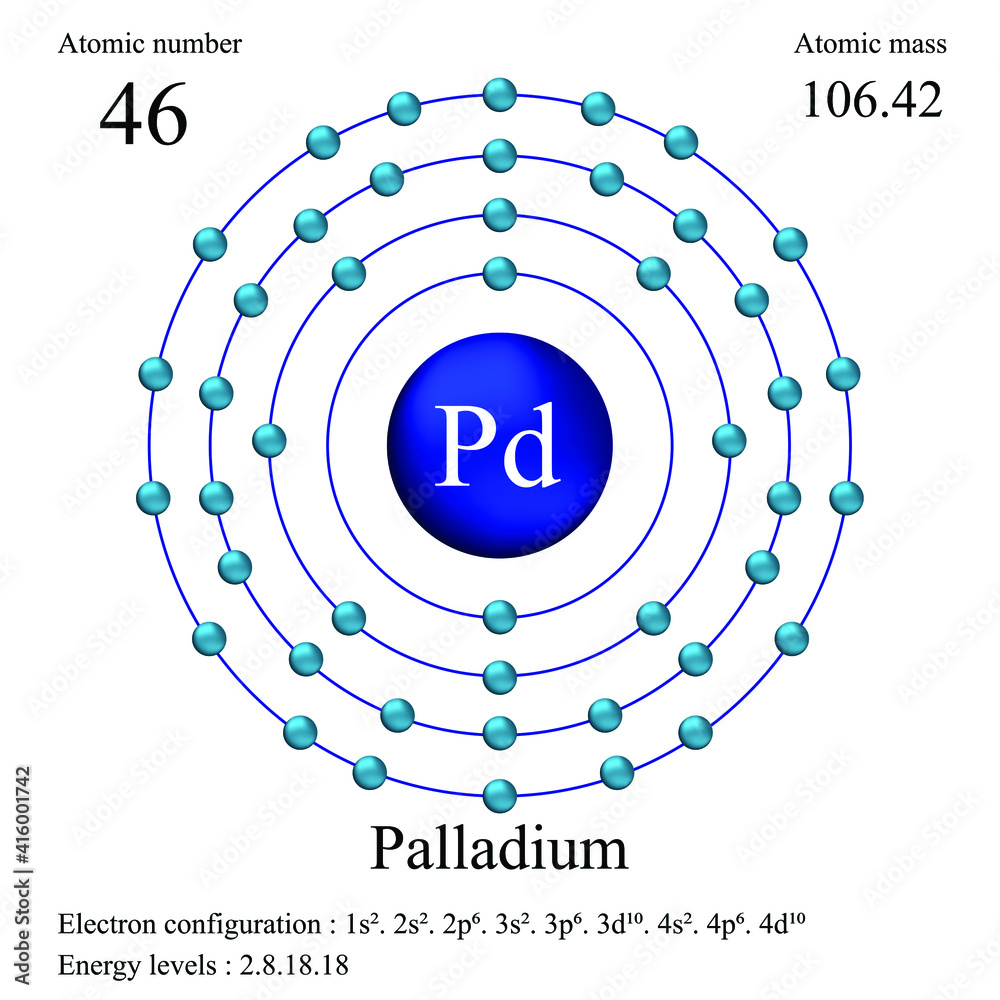
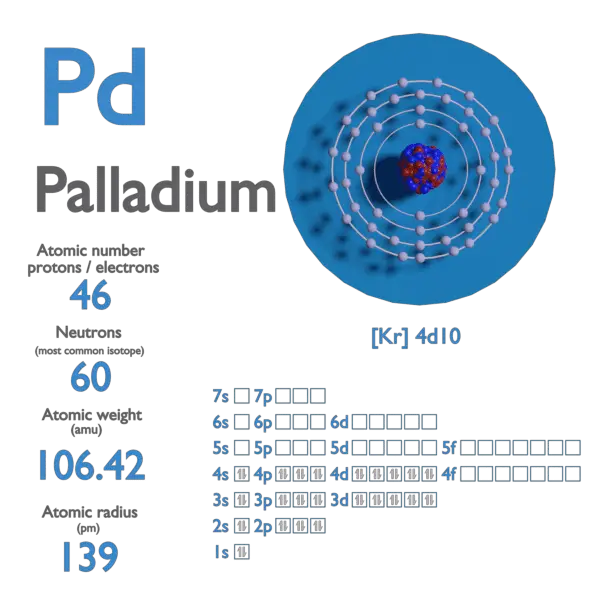
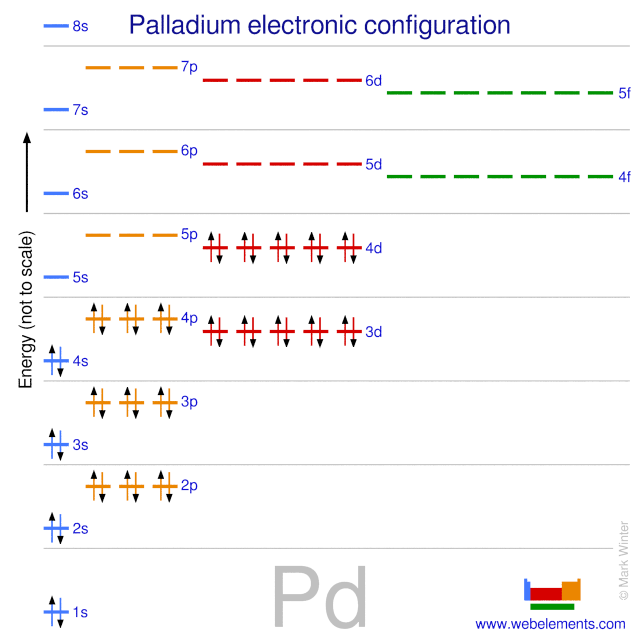
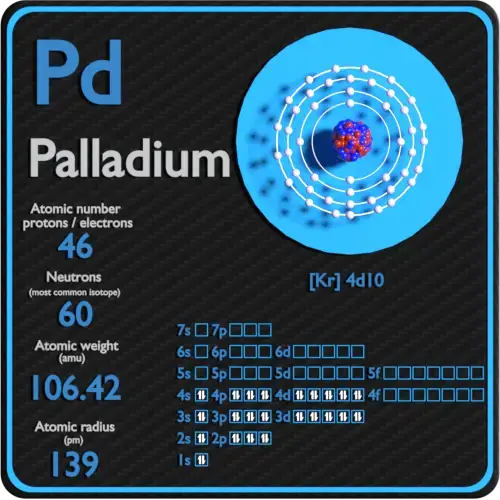
Palladium atomic structure has atomic number, atomic mass, electron configuration and energy levels. Stock Vector | Adobe Stock
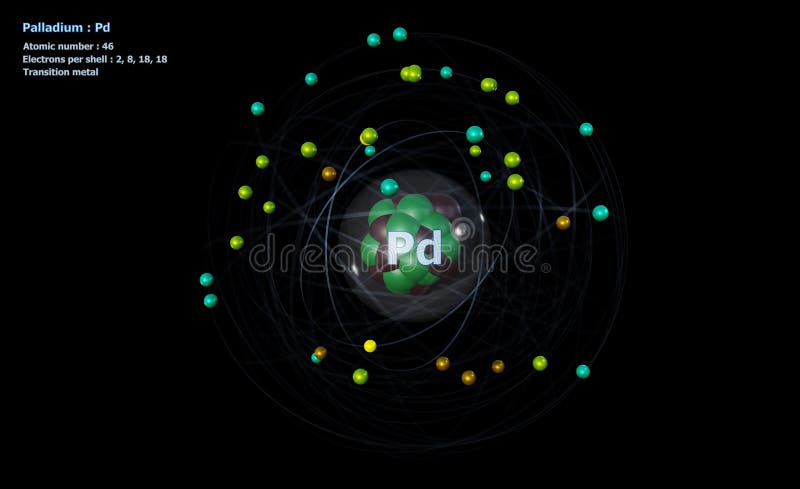
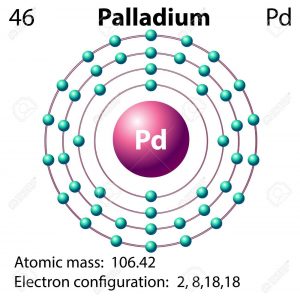
Atom of Palladium with Core and 46 Electrons on Black Stock Illustration - Illustration of scheme, nuclear: 251707411

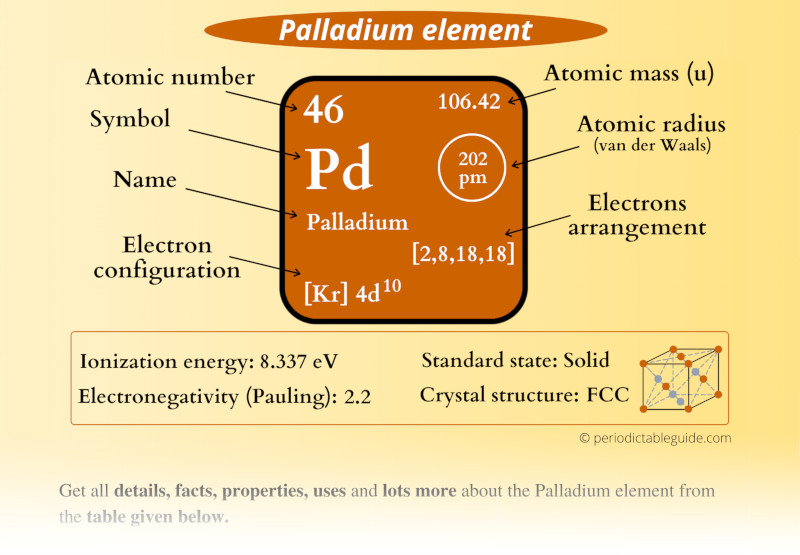
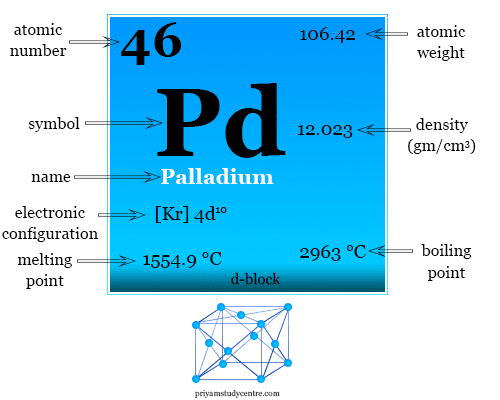
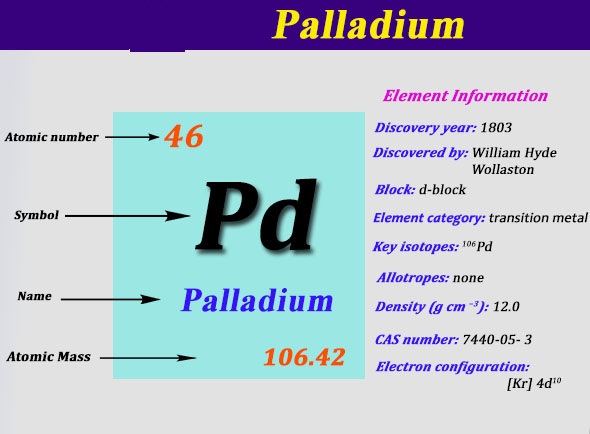
Pd Palladium Element Information: Facts, Properties, Trends, Uses and comparison - Periodic Table of the Elements | SchoolMyKids
![SOLVED: [Rectonces] In the ground state of 'palladium, Pd how many electrons have € = 1 as one of their quantum numbers? electrons how many electrons have n = 3as one of SOLVED: [Rectonces] In the ground state of 'palladium, Pd how many electrons have € = 1 as one of their quantum numbers? electrons how many electrons have n = 3as one of](https://cdn.numerade.com/ask_images/474a78b43f504f49b6e5104d89edea8b.jpg)