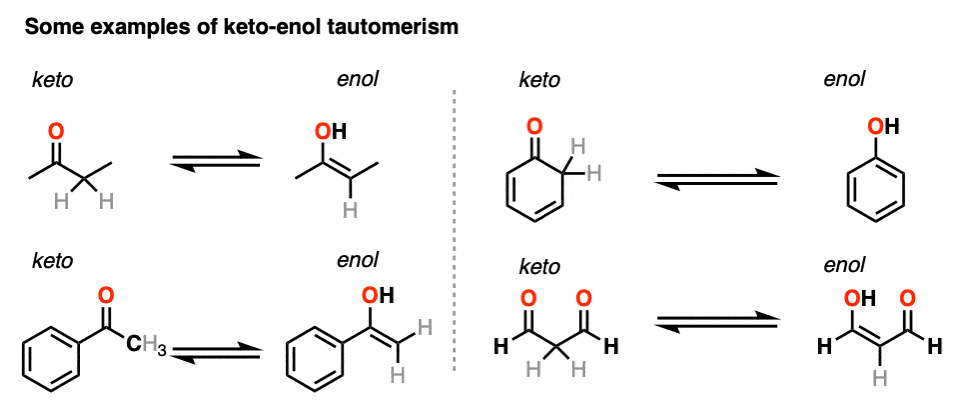
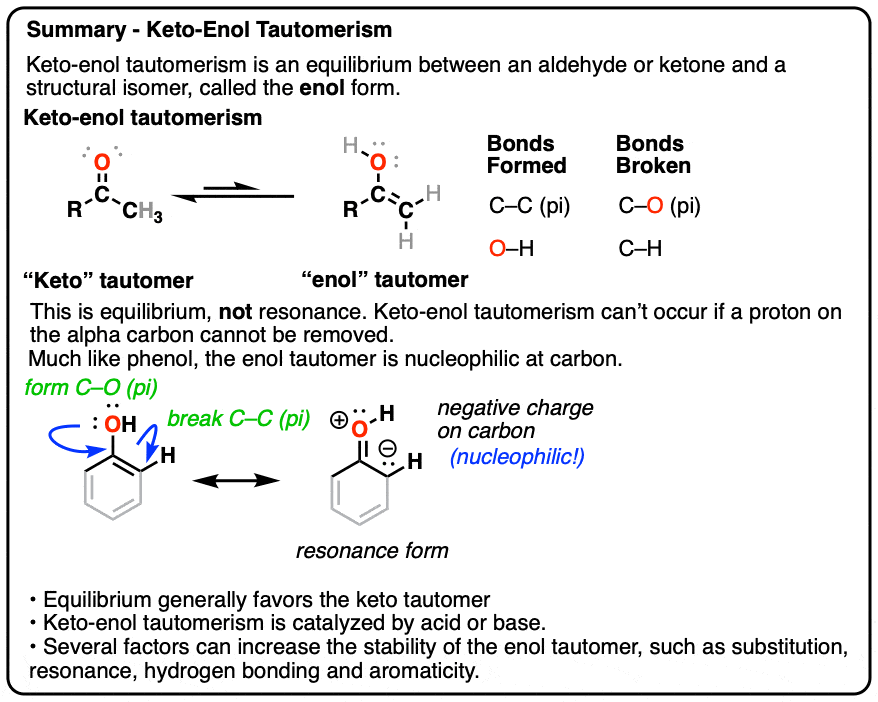
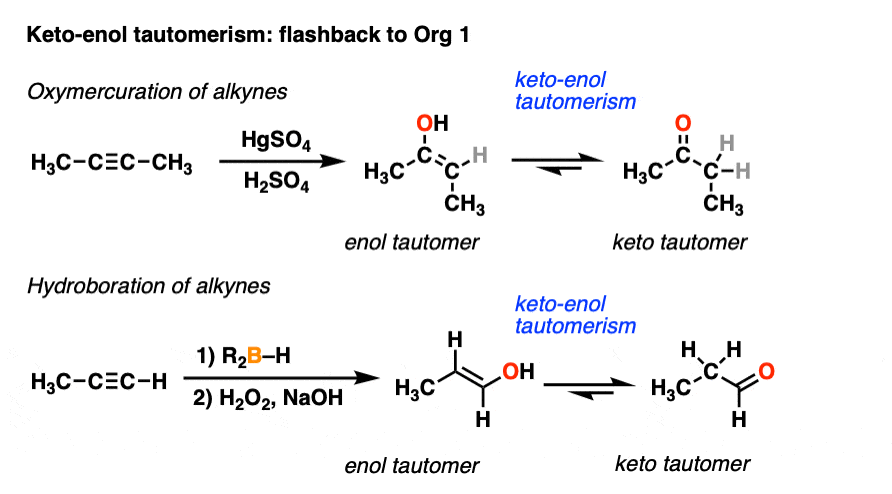
![15N, 13C and 1H NMR study of tautomerism and E/Z isomerism in 3-[(Z)-(2-phenylhydrazinylidene)methyl]quinoxalin-2(1H)-one and 3-[(E)-(2-phenylhydrazinylidene)methyl]quinoxalin-2(1H)-one - ScienceDirect 15N, 13C and 1H NMR study of tautomerism and E/Z isomerism in 3-[(Z)-(2-phenylhydrazinylidene)methyl]quinoxalin-2(1H)-one and 3-[(E)-(2-phenylhydrazinylidene)methyl]quinoxalin-2(1H)-one - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0143720818326780-fx1.jpg)
15N, 13C and 1H NMR study of tautomerism and E/Z isomerism in 3-[(Z)-(2-phenylhydrazinylidene)methyl]quinoxalin-2(1H)-one and 3-[(E)-(2-phenylhydrazinylidene)methyl]quinoxalin-2(1H)-one - ScienceDirect

Voltage and temperature dependence of the junction conductance.: (a)... | Download Scientific Diagram

Enol Forms of 1,3‐Indanedione, Their Stabilization by Strong Hydrogen Bonding, and Zwitterion‐Assisted Interconversion - Sigalov - 2010 - European Journal of Organic Chemistry - Wiley Online Library

Temperature-Dependent Formation of Acetophenone Oligomers Accompanied by Keto–Enol Tautomerism: Real Space Distribution | The Journal of Physical Chemistry C
Temperature-dependence of the 1 H NMR chemical shifts of the aromatic... | Download Scientific Diagram
![Energies | Free Full-Text | Using Chou's 5-Step Rule to Evaluate the Stability of Tautomers: Susceptibility of 2-[(Phenylimino)-methyl]-cyclohexane-1,3-diones to Tautomerization Based on the Calculated Gibbs Free Energies Energies | Free Full-Text | Using Chou's 5-Step Rule to Evaluate the Stability of Tautomers: Susceptibility of 2-[(Phenylimino)-methyl]-cyclohexane-1,3-diones to Tautomerization Based on the Calculated Gibbs Free Energies](https://pub.mdpi-res.com/energies/energies-13-00183/article_deploy/html/images/energies-13-00183-ag-550.jpg?1579175166)
Energies | Free Full-Text | Using Chou's 5-Step Rule to Evaluate the Stability of Tautomers: Susceptibility of 2-[(Phenylimino)-methyl]-cyclohexane-1,3-diones to Tautomerization Based on the Calculated Gibbs Free Energies
![Energies | Free Full-Text | Using Chou's 5-Step Rule to Evaluate the Stability of Tautomers: Susceptibility of 2-[(Phenylimino)-methyl]-cyclohexane-1,3-diones to Tautomerization Based on the Calculated Gibbs Free Energies Energies | Free Full-Text | Using Chou's 5-Step Rule to Evaluate the Stability of Tautomers: Susceptibility of 2-[(Phenylimino)-methyl]-cyclohexane-1,3-diones to Tautomerization Based on the Calculated Gibbs Free Energies](https://pub.mdpi-res.com/energies/energies-13-00183/article_deploy/html/images/energies-13-00183-g003.png?1579175166)
Energies | Free Full-Text | Using Chou's 5-Step Rule to Evaluate the Stability of Tautomers: Susceptibility of 2-[(Phenylimino)-methyl]-cyclohexane-1,3-diones to Tautomerization Based on the Calculated Gibbs Free Energies
![Keto-enol tautomerism of (E)-2-[(3,4-dimethylphenylimino)methyl]-4-nitrophenol: Synthesis, X-ray, FT-IR, UV–Vis, NMR and quantum chemical characterizations - ScienceDirect Keto-enol tautomerism of (E)-2-[(3,4-dimethylphenylimino)methyl]-4-nitrophenol: Synthesis, X-ray, FT-IR, UV–Vis, NMR and quantum chemical characterizations - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0022286016308109-fx1.jpg)
Keto-enol tautomerism of (E)-2-[(3,4-dimethylphenylimino)methyl]-4-nitrophenol: Synthesis, X-ray, FT-IR, UV–Vis, NMR and quantum chemical characterizations - ScienceDirect
![Facile Conversion of syn‐[FeIV(O)(TMC)]2+ into the anti Isomer via Meunier's Oxo–Hydroxo Tautomerism Mechanism - Prakash - 2019 - Angewandte Chemie International Edition - Wiley Online Library Facile Conversion of syn‐[FeIV(O)(TMC)]2+ into the anti Isomer via Meunier's Oxo–Hydroxo Tautomerism Mechanism - Prakash - 2019 - Angewandte Chemie International Edition - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/bfd4b6e7-e975-46f3-891a-252364a75505/anie201811454-toc-0001-m.jpg)
Facile Conversion of syn‐[FeIV(O)(TMC)]2+ into the anti Isomer via Meunier's Oxo–Hydroxo Tautomerism Mechanism - Prakash - 2019 - Angewandte Chemie International Edition - Wiley Online Library

Reversible Shifting of a Chemical Equilibrium by Light: The Case of Keto–Enol Tautomerism of a β-Ketoester | Organic Letters

Substituent, Temperature and Solvent Effects on the Keto-Enol EQUILIBRIUM in <i>β</i>-Ketoamides: A Nuclear Magnetic Resonance Study

Temperature dependence of the rate constants k 1 and k 2. A ln k 1 as a... | Download Scientific Diagram

Base-Pairing, Tautomerism, and Mismatch Discrimination of 7-Halogenated 7-Deaza-2'-deoxyisoguanosine: Oligonucleotide Duplexes with Parallel and Antiparallel Chain Orientation | Journal of the American Chemical Society