Kal(so4)2 12h2o Potash Alum Potassium Alum Stone - Buy Potassium Alum Stone,Potash Alum Stone,Kal(so4)2 12h2o Potassium Alum Stone Product on Alibaba.com
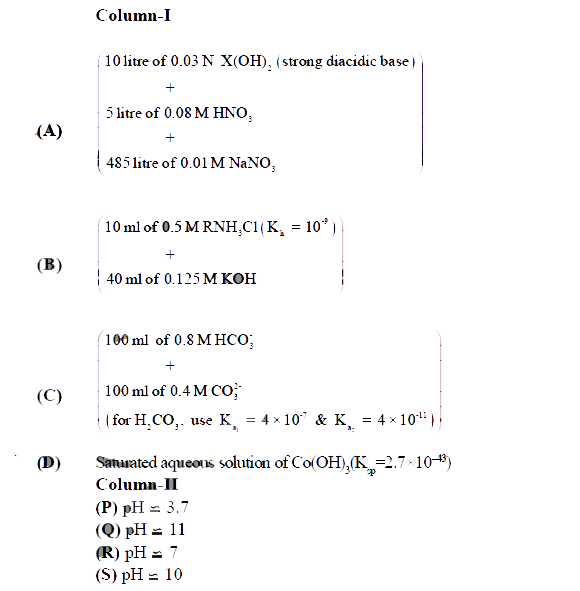
Potash alum is K Al(SO4)2. 12H2O. As a strong eletrolyte, it is considered to be 100% dissociated into K^+, Al^(3+) and SO4^(2-). The solution is acidic because of the hydrolysis of Al^(3+),

reactionchamber on Twitter: "Potassium alum is the double sulfate salt of potassium and aluminium. It is commonly encountered as the dodecahydrate, KAl(SO4)2·12H2O. It crystallizes in an octahedral structure in neutral solution. #alum #

Kal(so4)2.12h2o Aluminium Potassium Sulfate Potassium Alum Powder - Buy Potassium Alum Powder,Aluminium Potassium Sulfate Powder,Kal(so4)2 12h2o Potassium Alum Product on Alibaba.com

Kal(so4)2 12h2o Aluminum Potassium Sulfate Lump Potassium Alum Potash Alum - Buy Potash Alum,Aluminum Potassium Sulfate,Kal(so4)2 12h2o Potash Alum Product on Alibaba.com
Cubic structure of alum K(Al,Cr)(SO 4 ) 2 ⋅12H 2 O, space group Pa3 _ .... | Download Scientific Diagram

PPT - Exp 12 Synthesis: Preparation of Alum Alum: potassium aluminum sulfate dodecahydrate PowerPoint Presentation - ID:5409973

SOLVED: To synthesize alum, KAl(SO4)2 • 12H2O, 1.39 g of solid aluminum are reacted with excess potassium hydroxide and sulfuric acid as described in the experimental section of the writeup in the

Kal(so4)2 12h2o Aluminium Potassium Sulfate Potash Alum Price - Buy Potash Alum Price,Aluminium Potassium Sulfate Price,Kal(so4)2 12h2o Product on Alibaba.com

SOLVED: To synthesize alum, KAl(SO4)2 • 12H2O, 1.39 g of solid aluminum are reacted with excess potassium hydroxide and sulfuric acid as described in the experimental section of the writeup in the
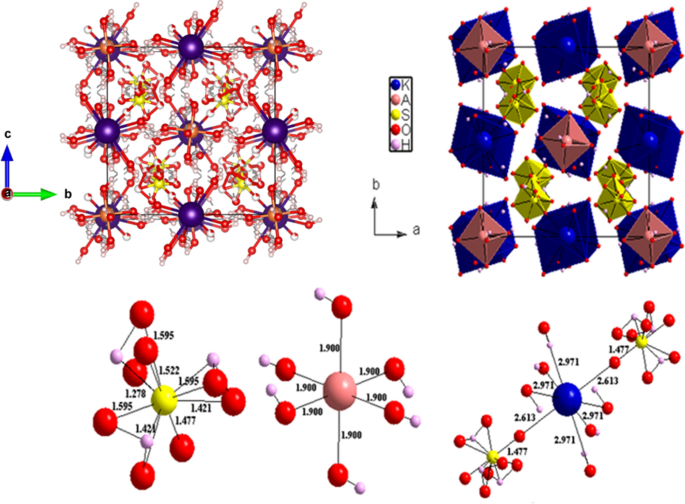
Investigations on KAl(SO4)2∙12H2O: A Candidate α-Alum Material for Energy Storage Applications | SpringerLink