New UK medical device regulation spells potential trial concerns for some IVD players - Medical Device Network
CD33 antibodies | CE-IVD reagents | Clinical flow cytometry | Cell manufacturing platform | Products | Miltenyi Biotec | Great Britain

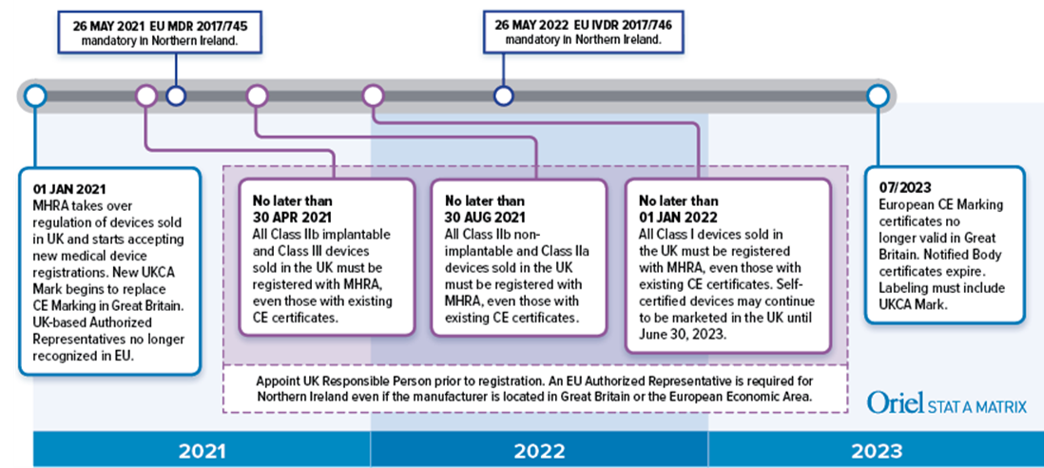
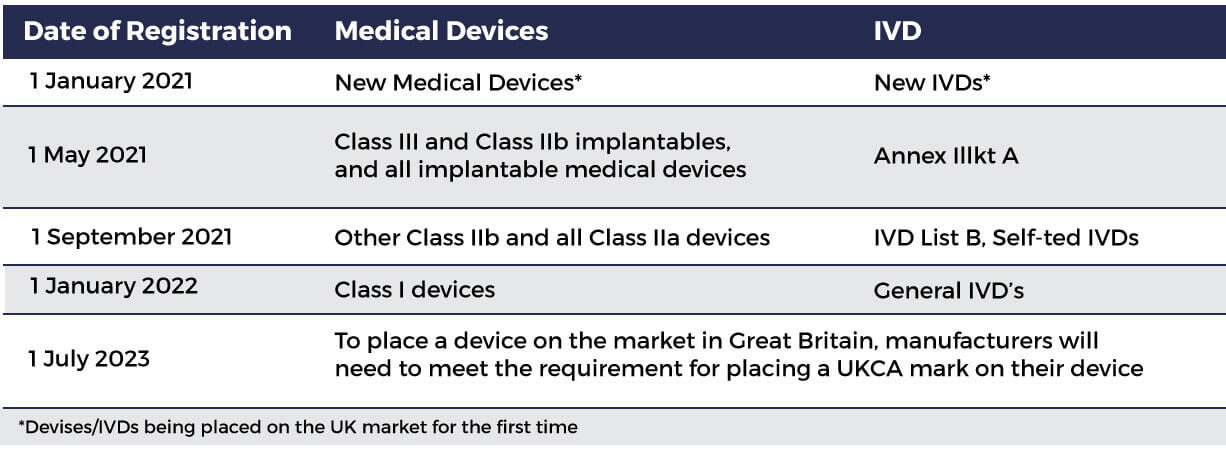
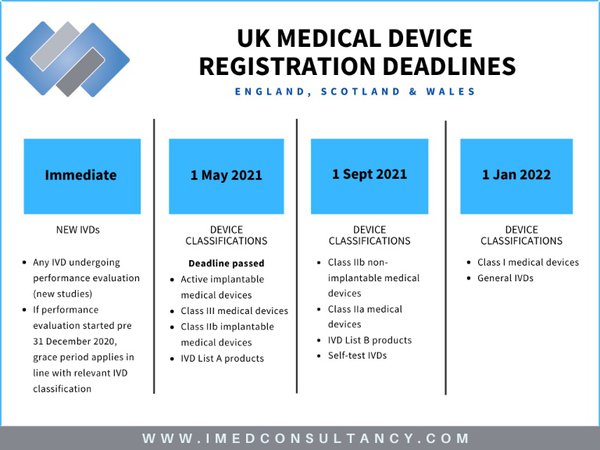
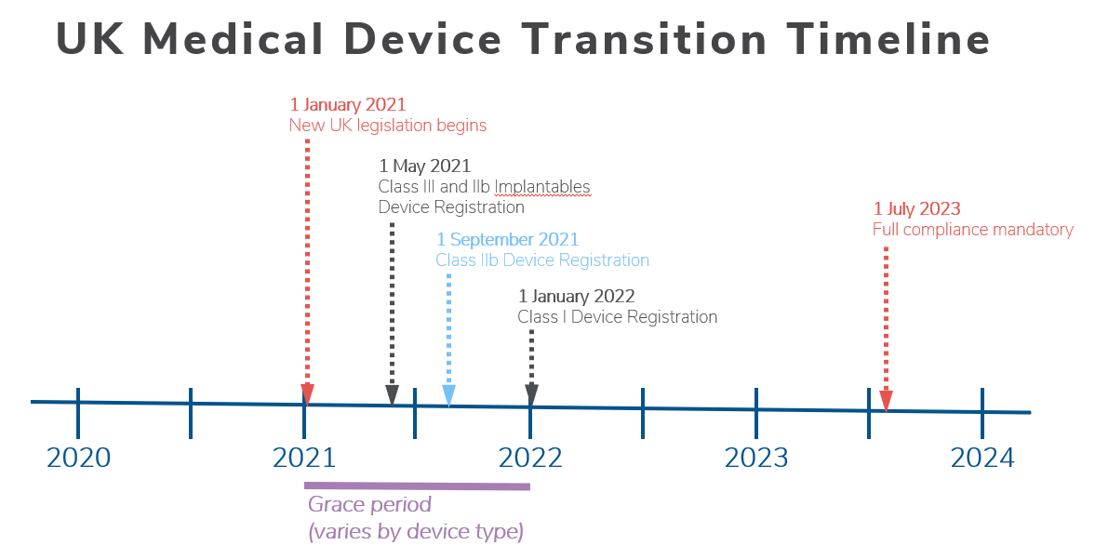
MHRA guidance on registration and deadlines for medical devices and IVDs in Great Britain and Northern Ireland
CD10 antibodies | CE-IVD reagents | Clinical flow cytometry | Cell manufacturing platform | Products | Miltenyi Biotec | Great Britain

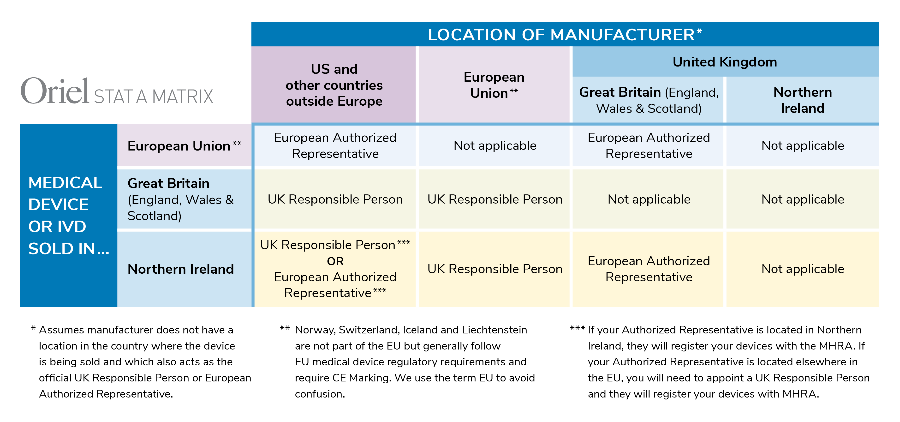
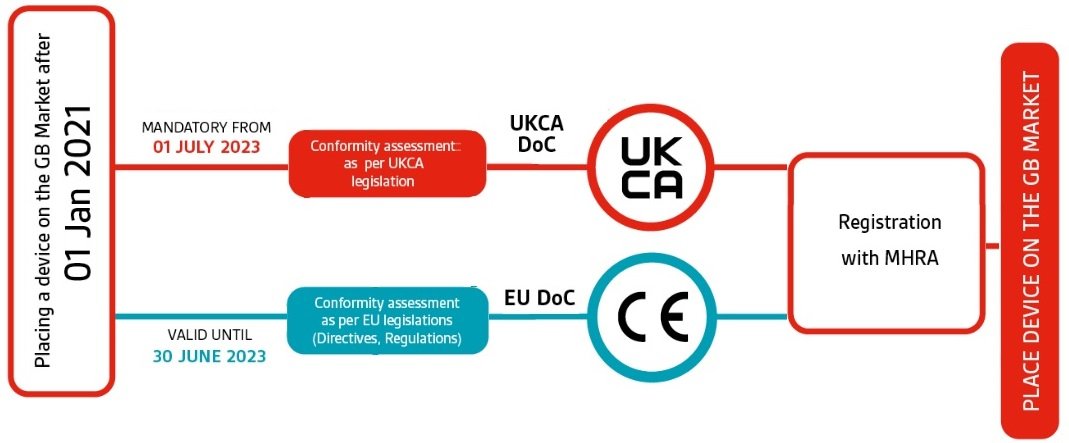
Top 10 Questions regarding the UK Responsible Person and medical device/IVD registration with the MHRA
Top 10 Questions regarding the UK Responsible Person and medical device/IVD registration with the MHRA