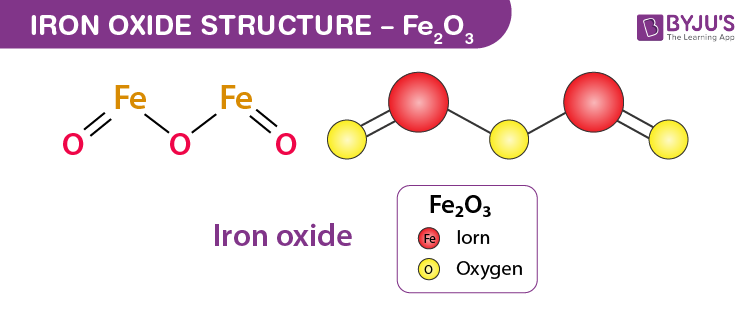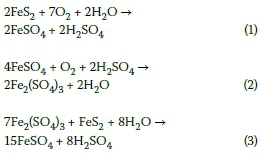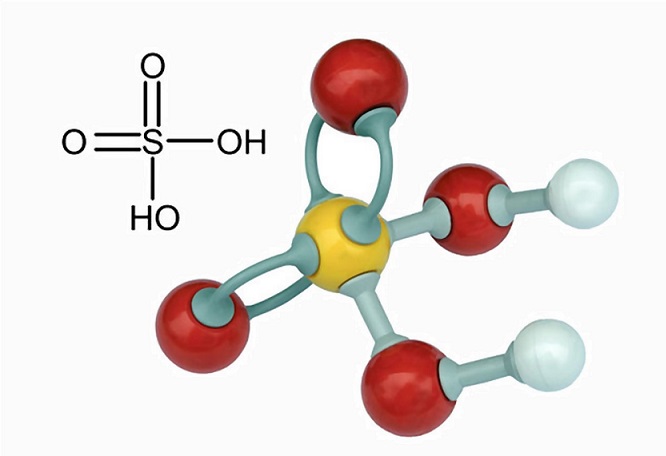
Calculate the equivalent mass of sulphuric acid (ii) The reaction between aluminium and ferric oxide can generate temperatures up to 3273 K and is used in welding metals. (Atomic mass of AI=

The Oxidation of Fe(II) in Acidic Sulfate Solutions with Air at Elevated Pressures. Part 1. Kinetics above 1 M H2SO4 | Industrial & Engineering Chemistry Research
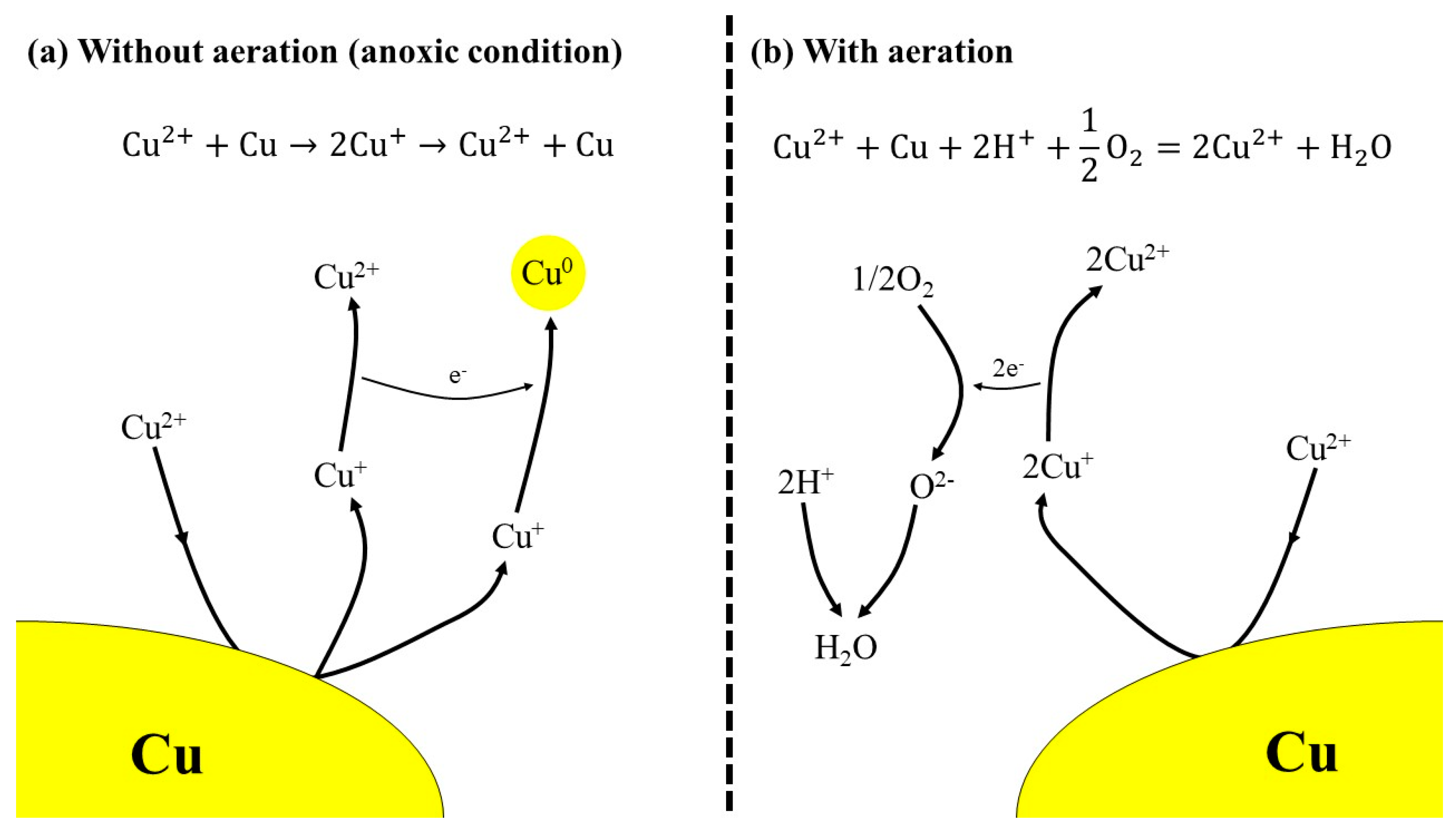
Metals | Free Full-Text | Improvement of Copper Metal Leaching in Sulfuric Acid Solution by Simultaneous Use of Oxygen and Cupric Ions

The process of pyrite weathering in a deep metal mine. Four general... | Download Scientific Diagram

7.2 Making Salts What is made when acids and metals react? What is made when alkalis and metals react? 27 August 2015 Bonneville salt flats in America. - ppt download

Write word equations and then balanced equations for the reaction taking place when:(a) Dilute sulphuric acid reacts with zinc granules.(b) Dilute hydrochloric acid reacts with magnesium ribbon.(c) Dilute sulphuric acid reacts with

THE RICO-ARGENTINE CO. SULPHURIC ACID MILL. ALTHOUGH THE MILL HAS BEEN CLOSED FOR SIX YEARS, WATER SEEPING FROM OLD MINE SHAFTS POLLUTES NEARBY STREAMS WITH IRON OXIDE Stock Photo - Alamy

Explain with the help of chemical equations what happens when: - (a) Copper is heated strongly in air.(b) Iron is reached with steam.(c) Sodium is reacted with dilute sulphuric acid.(d) Zinc is

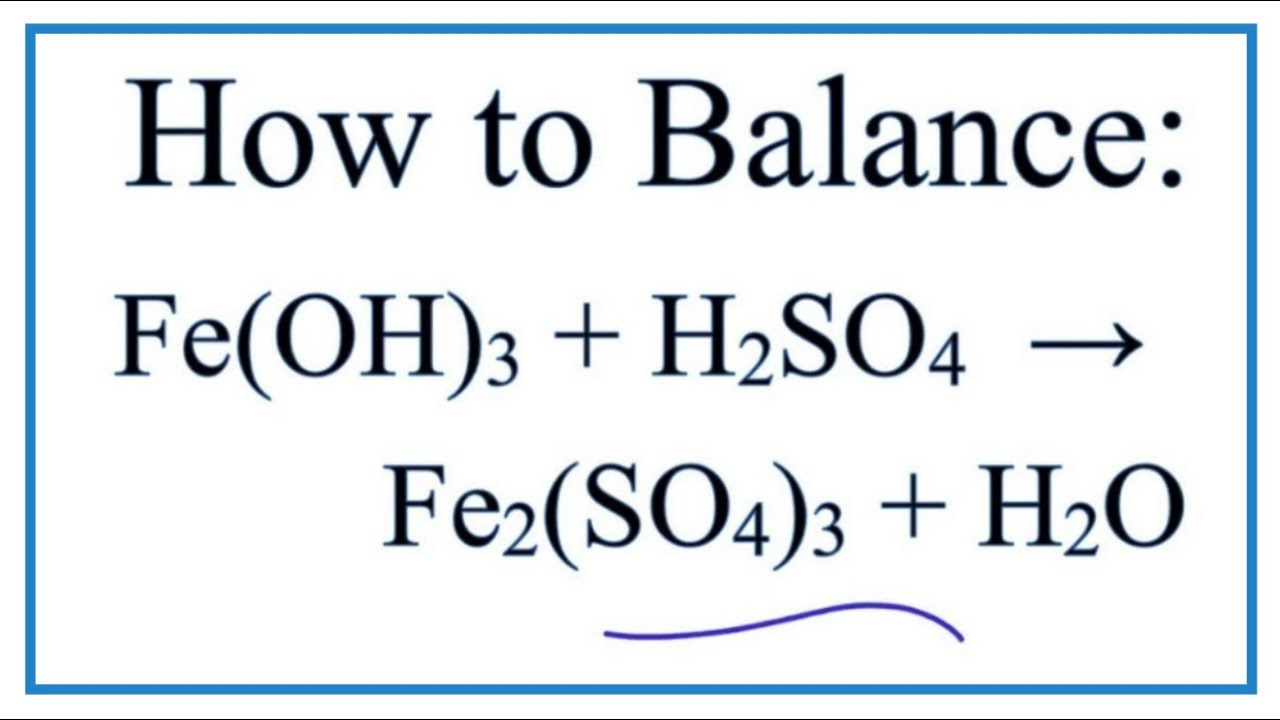
ferric oxide reacts with sulphuric acid to give ferric sulphate and water balance the equation - Brainly.in

Causes chemistry of rusting rust prevention introduction to oxidation reduction REDOX reactions gcse igcse KS4 science chemistry revision notes revising

A Facile One‐Pot Transformation of Aromatic Aldehydes/Ketones to Amides: Fe2O3@SiO2 as an Environmentally Benign Core‐Shell Catalyst - Jain - 2018 - ChemistrySelect - Wiley Online Library

How to Balance Fe + H2SO4 = FeSO4 + Fe2(SO4)3 + H2O + SO2 (Iron + Concentrated Sulfuric acid) - YouTube