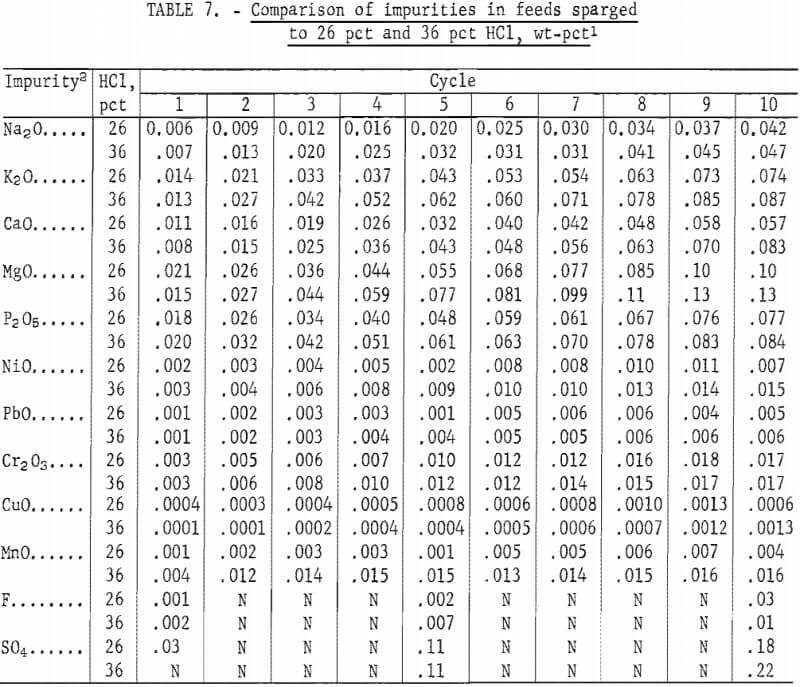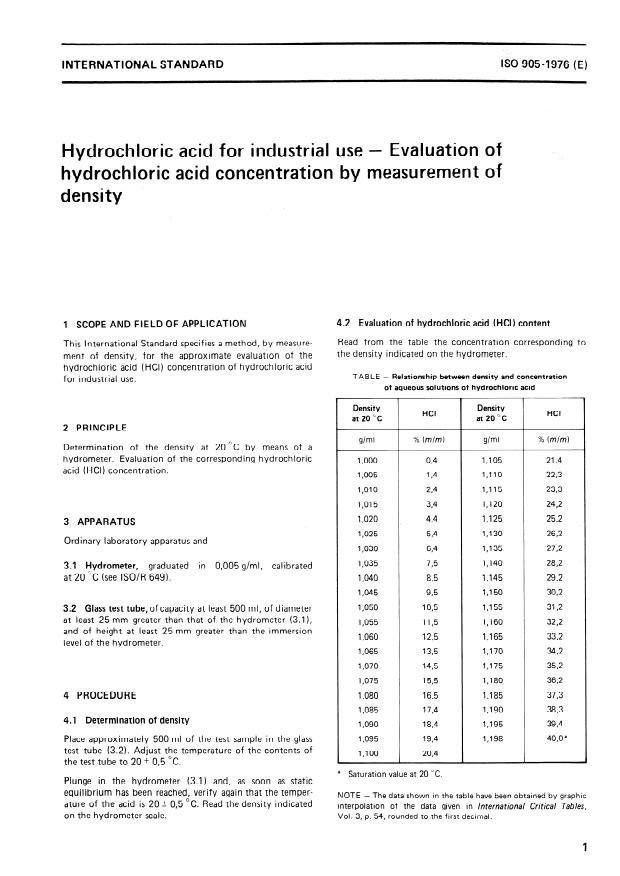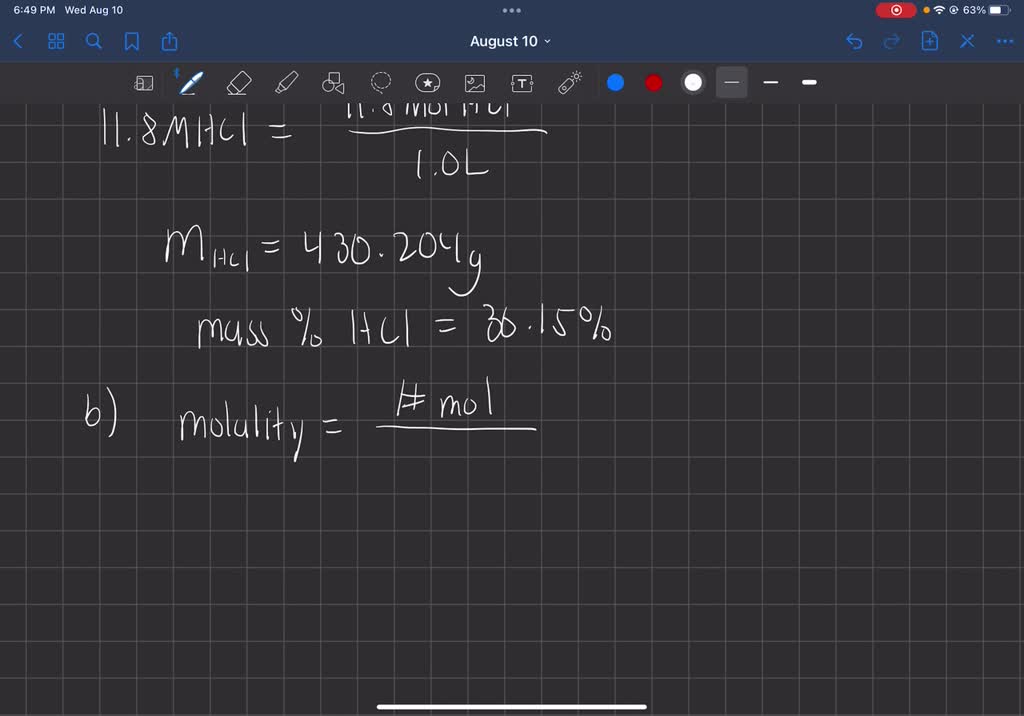
SOLVED: Commercial concentrated Hydrochloric acid is 11.8 M HCl and has a density Of 1.190 g/mL. Calculate the: a. mass percent HCI b. Molality c. mole fraction of HCI

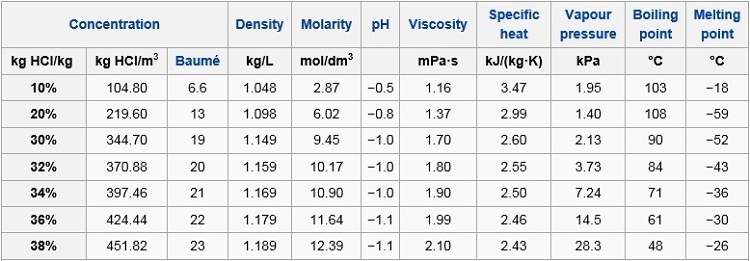
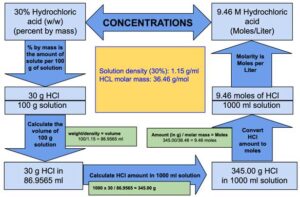
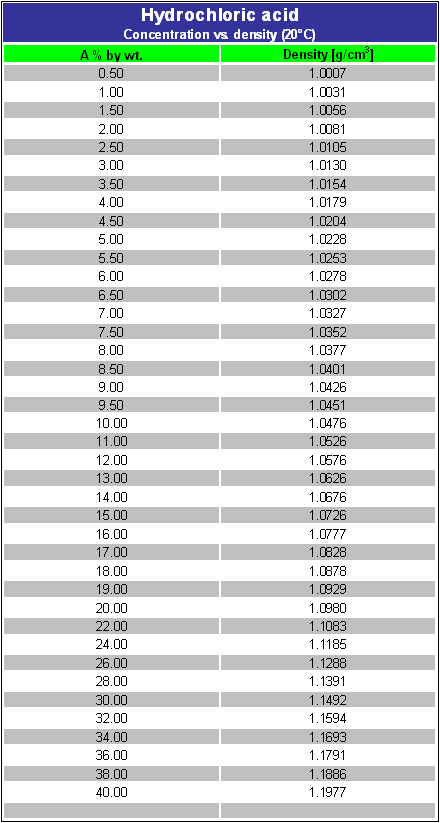
Concentration hydrochloric acid has 38% of HCl by weight with a density of 1.1885 g per ml. Calculate the molarity of the acid. What volume of the acid on dilution to one
A certain sample of concentrated hydrochloric acid contains 50% HCl by mass and has a density of 1.20 g/cm^3. What is the molarity of this sample? - Quora
Concentrated hydrochloric acid contains 37% (by mass) HCl. The density of its solution is 1.18 g/ml.

SOLVED: What is the mole fraction of hydrochloric acid in a 7.20% (bY mass) aqueous solution? 0.0383 0.0185 0.0369 The density of the solution is needed to solve the problem: 0.0739
An aqueous solution is 40.0% by mass hydrochloric acid, HCl, and has a density of 1.20 g/mL. What is the molarity of hydrochloric acid in the solution? - Quora