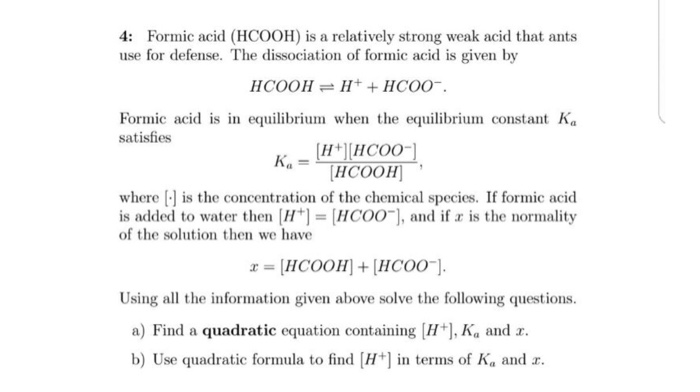
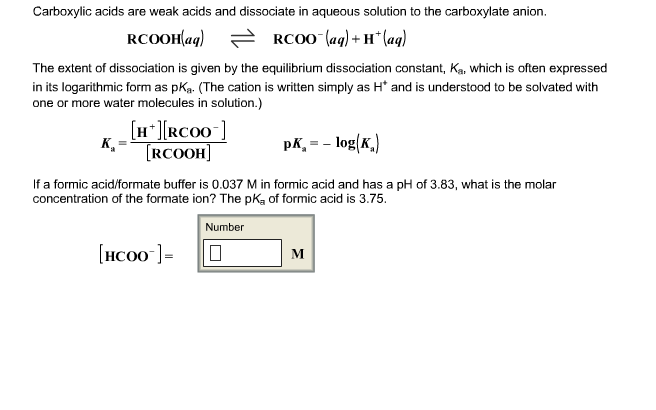
What is the pH of a 0.0944 M aqueous solution of formic acid, HCOOH? (Ka = 1.8 x 10-4) | Homework.Study.com

At certain temperature, dissociation constant of formic acid and acetic acid are 1.8xx10^(-4) and 1.8xx10^(-5) respectively. At what concentration of acetic solution, the H93)O^(+) ion concentration is same as that in 0.001

Acid Dissociation Constant. Dissociation Constants For a generalized acid dissociation, the equilibrium expression would be This equilibrium constant. - ppt download

Secondary hydrogen isotope effect in the dissociation constant of formic acid - Transactions of the Faraday Society (RSC Publishing)

The concentration of hydrogen ions in a 0.2 M solution of formic acid is 6.4 × 10^-3mol L^-1 . To this solution, sodium formate is added so as to adjust the concentration

The dissociation constants of formic and acetic acids are 1.77 × 10^-4 and 1.75 × 10^-5 , respectively
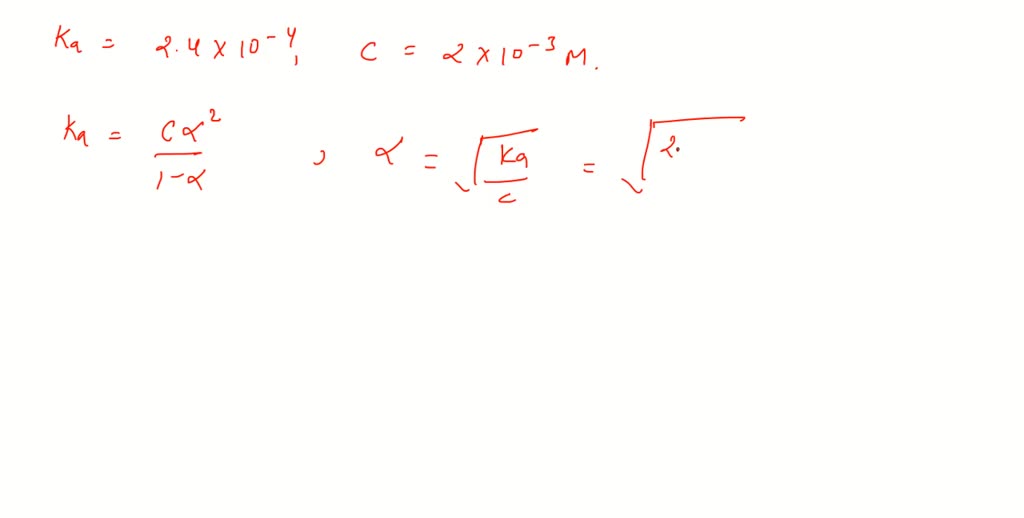
SOLVED:The dissociation constant of formic acid is 0.00024. The hydrogen ion concentration in 0.002 M-HCOOH solution is nearly (a) 6.93 ×10^-4 M (b) 4.8 ×10^-7 M (c) 5.8 ×10^-4 M (d) 1.4 ×10^-4 M

The ionization constant of formic acid is `1.8xx10^(-4)`. Around what pH will its mixture with - YouTube
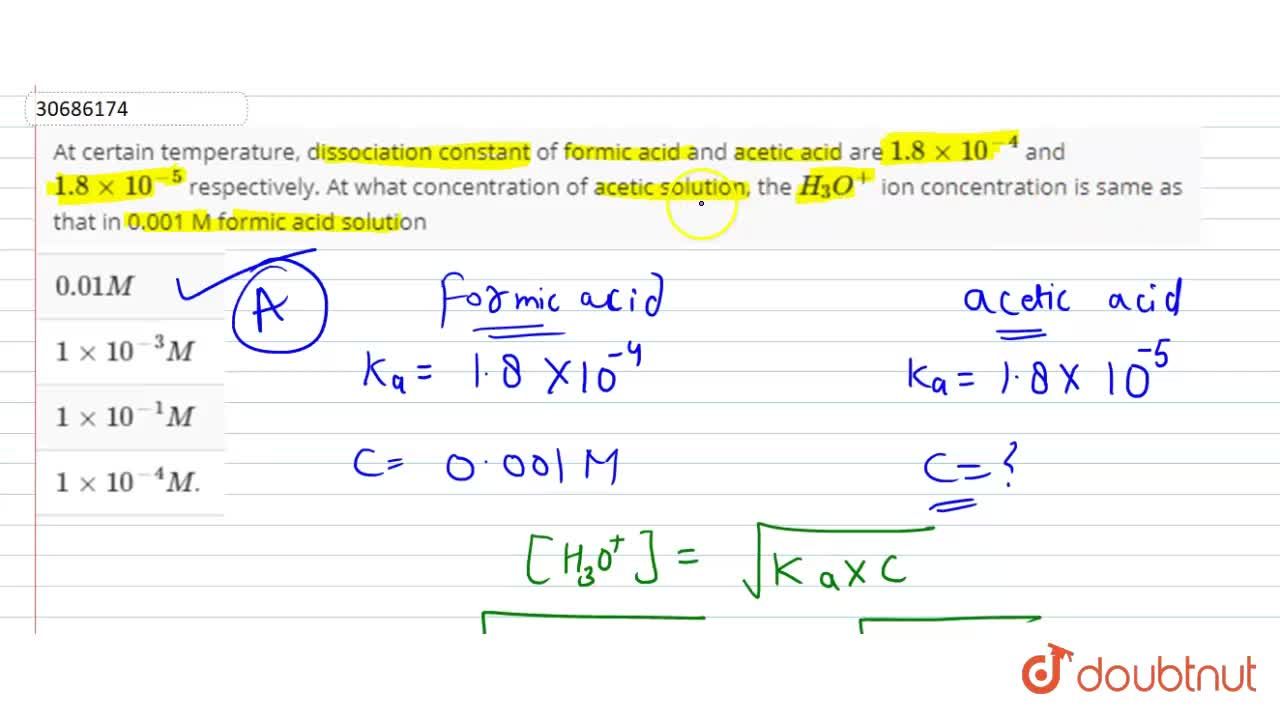
At certain temperature, dissociation constant of formic acid and acetic acid are 1.8xx10^(-4) and 1.8xx10^(-5) respectively. At what concentration of acetic solution, the H93)O^(+) ion concentration is same as that in 0.001

Value of dissociation constant of acetic acid is 10^-6 , where as dissociation constant of formic acid is 10^-5 . Which of the following will be the value of pKa (acetic acid) - pKa (formic acid)?
![The self ionization constant for pure formic acid, K = [HCOOH^-2] [HCOO^ - ] has been estimated as 10^-6 at room temperature. The density of formic acid is 1.22 g/cm^3 . The The self ionization constant for pure formic acid, K = [HCOOH^-2] [HCOO^ - ] has been estimated as 10^-6 at room temperature. The density of formic acid is 1.22 g/cm^3 . The](https://dwes9vv9u0550.cloudfront.net/images/11469763/0c5bdcb3-4186-4faf-92ed-af469406afbf.jpg)
The self ionization constant for pure formic acid, K = [HCOOH^-2] [HCOO^ - ] has been estimated as 10^-6 at room temperature. The density of formic acid is 1.22 g/cm^3 . The

Formic acid dimer dissociation enthalpy as a function of temperature... | Download Scientific Diagram