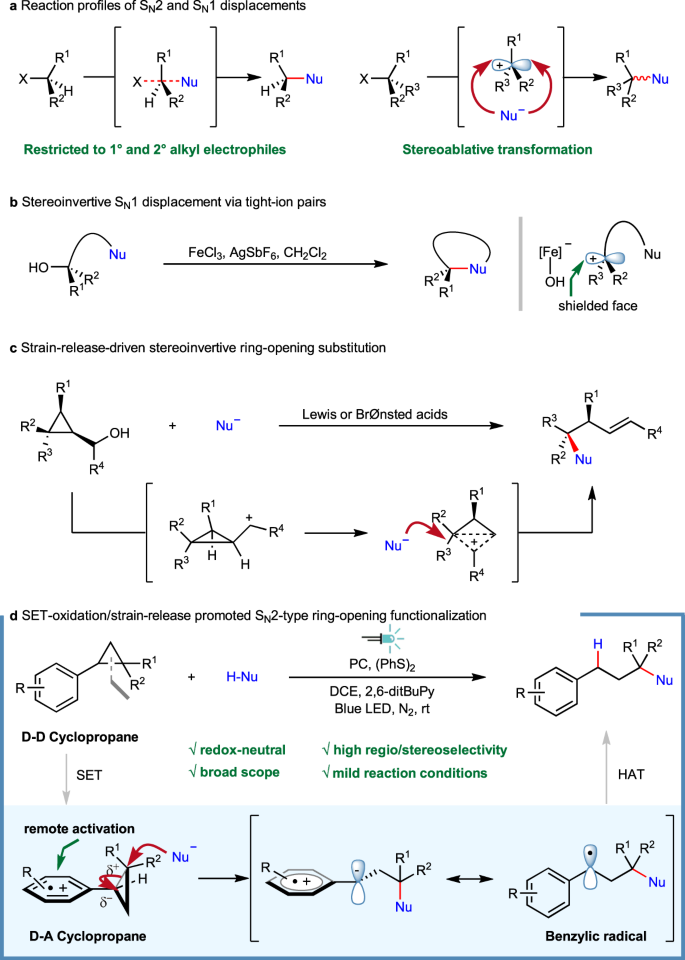
Photoredox-catalyzed C–C bond cleavage of cyclopropanes for the formation of C(sp3)–heteroatom bonds | Nature Communications
![Nucleophilic Ring Opening of Donor–Acceptor Cyclopropanes with the Cyanate Ion: Access to Spiro[pyrrolidone-3,3′-oxindoles] | The Journal of Organic Chemistry Nucleophilic Ring Opening of Donor–Acceptor Cyclopropanes with the Cyanate Ion: Access to Spiro[pyrrolidone-3,3′-oxindoles] | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/acs.joc.8b00922/asset/images/medium/jo-2018-00922v_0006.gif)
Nucleophilic Ring Opening of Donor–Acceptor Cyclopropanes with the Cyanate Ion: Access to Spiro[pyrrolidone-3,3′-oxindoles] | The Journal of Organic Chemistry

Nucleophilic Ring Opening of Donor–Acceptor Cyclopropanes Catalyzed by a Brønsted Acid in Hexafluoroisopropanol | Organic Letters
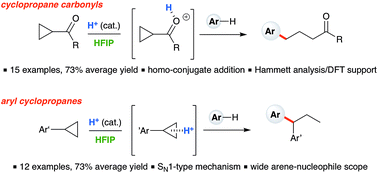
Ring-opening hydroarylation of monosubstituted cyclopropanes enabled by hexafluoroisopropanol - Chemical Science (RSC Publishing)
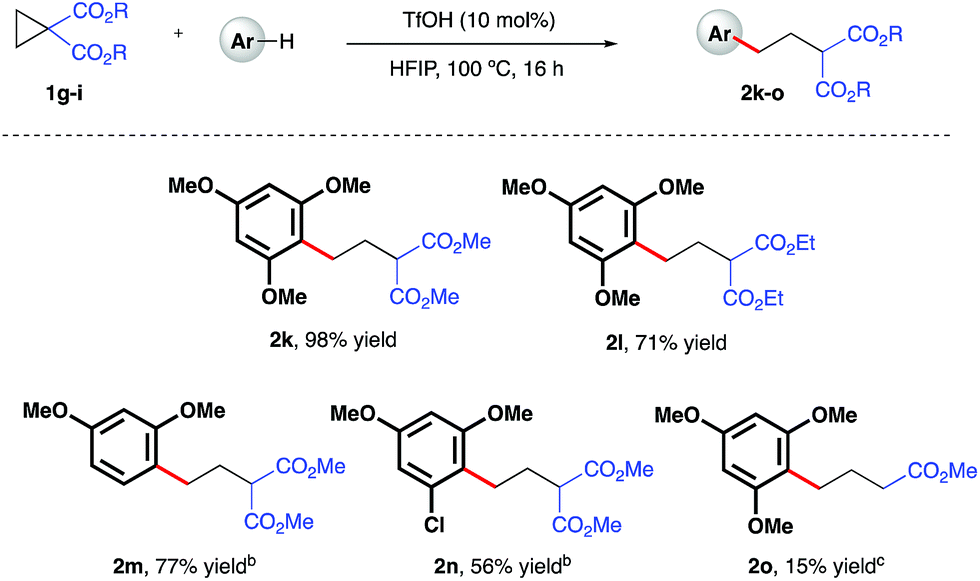
Ring-opening hydroarylation of monosubstituted cyclopropanes enabled by hexafluoroisopropanol - Chemical Science (RSC Publishing) DOI:10.1039/C8SC02126K
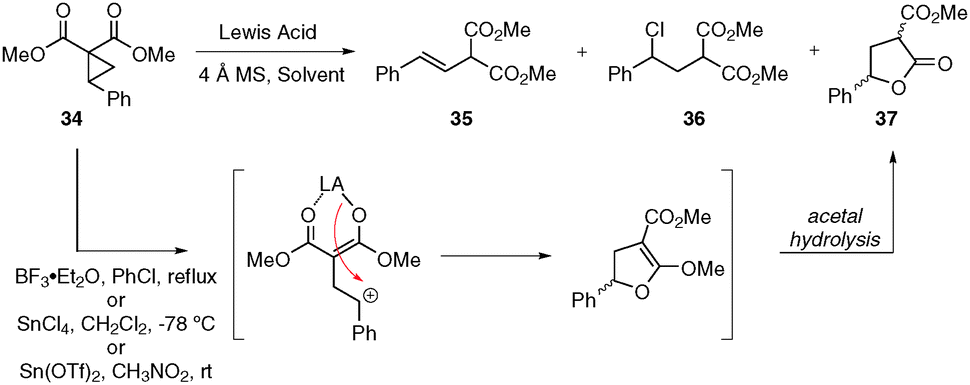
Intramolecular donor–acceptor cyclopropane ring-opening cyclizations - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C3CS60238A
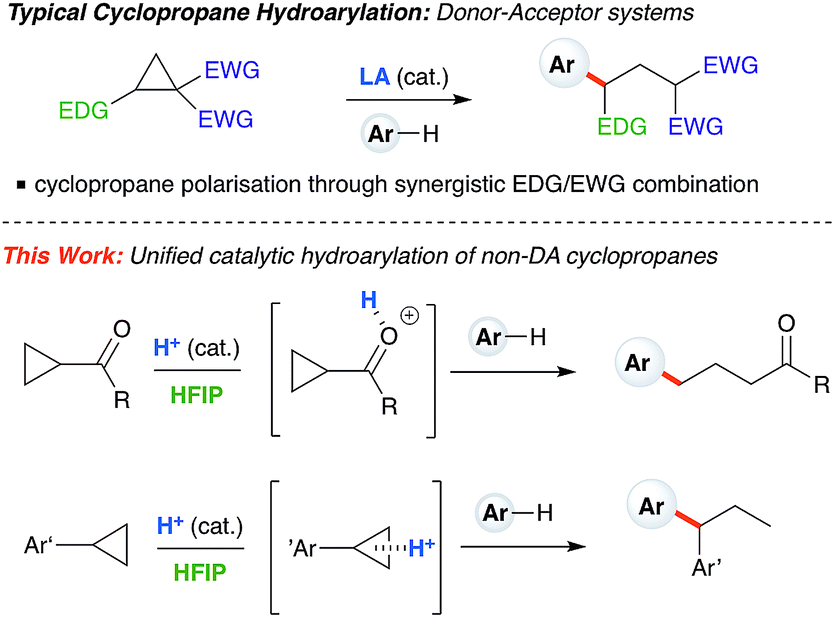
Ring-opening hydroarylation of monosubstituted cyclopropanes enabled by hexafluoroisopropanol - Chemical Science (RSC Publishing) DOI:10.1039/C8SC02126K
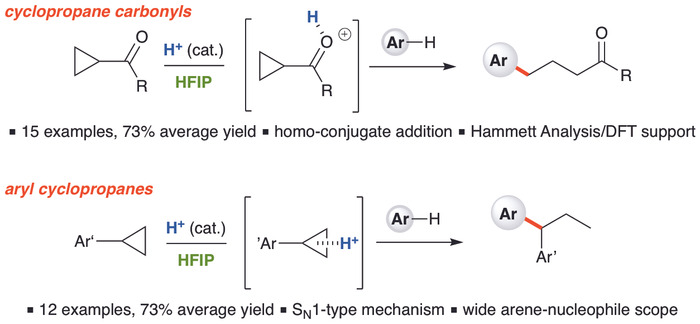
Ring-Opening Hydroarylation of Monosubstituted Cyclopropanes Enabled by Hexafluoroisopropanol | Organic Chemistry | ChemRxiv | Cambridge Open Engage

Scheme 3. The ring-opening of cyclopropane can either pass through a... | Download Scientific Diagram

Mild Ring‐Opening 1,3‐Hydroborations of Non‐Activated Cyclopropanes - Wang - 2018 - Angewandte Chemie International Edition - Wiley Online Library

Ring-opening hydroarylation of monosubstituted cyclopropanes enabled by hexafluoroisopropanol. - Abstract - Europe PMC
![Nucleophilic Ring Opening of Donor–Acceptor Cyclopropanes with the Cyanate Ion: Access to Spiro[pyrrolidone-3,3′-oxindoles] | The Journal of Organic Chemistry Nucleophilic Ring Opening of Donor–Acceptor Cyclopropanes with the Cyanate Ion: Access to Spiro[pyrrolidone-3,3′-oxindoles] | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/acs.joc.8b00922/asset/images/acs.joc.8b00922.social.jpeg_v03)