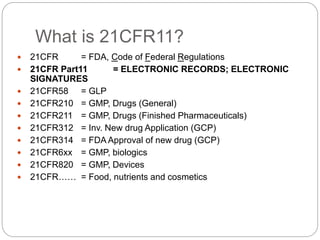
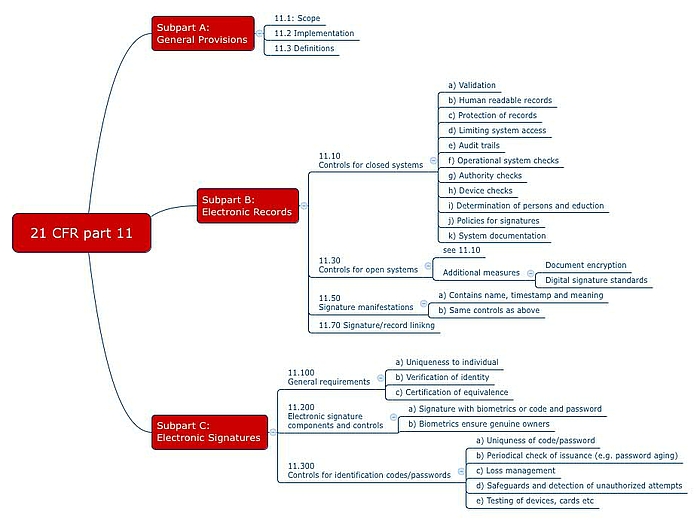
Enabling SharePoint for 21 CFR Part 11 Compliance - Electronic Signature Use Case - PDF Free Download

Title 21 of the Code of Federal Regulations Title 21 CFR Part 11 Food and Drug Administration, others, text, brand, title 21 Cfr Part 11 png | PNGWing

The CFR Title 21 Part 11, 210 & 211, 820 Memory Jogger (4 pack): Goal/QPC, Goal/QPC, Goal/QPC: 9781576812655: Amazon.com: Books

Book M2: 2022 Mini Pocket-Sized (3" x 5") Code of Federal Regulations – Clinical Research Resources, LLC

Book 23: 2022 Part 11 & Drug Development: Regulation, Preamble & FDA G – Clinical Research Resources, LLC





%20Complete%20Guide%20to%2021%20CFR%20Part%2011.png?width=250&height=324&name=(cover)%20Complete%20Guide%20to%2021%20CFR%20Part%2011.png)