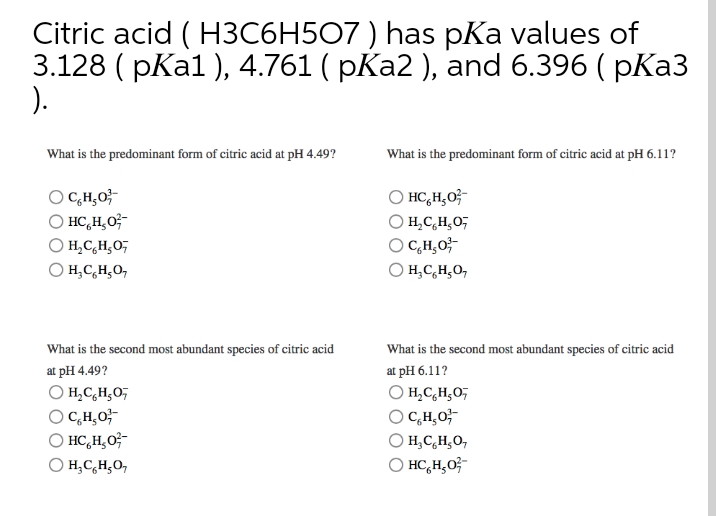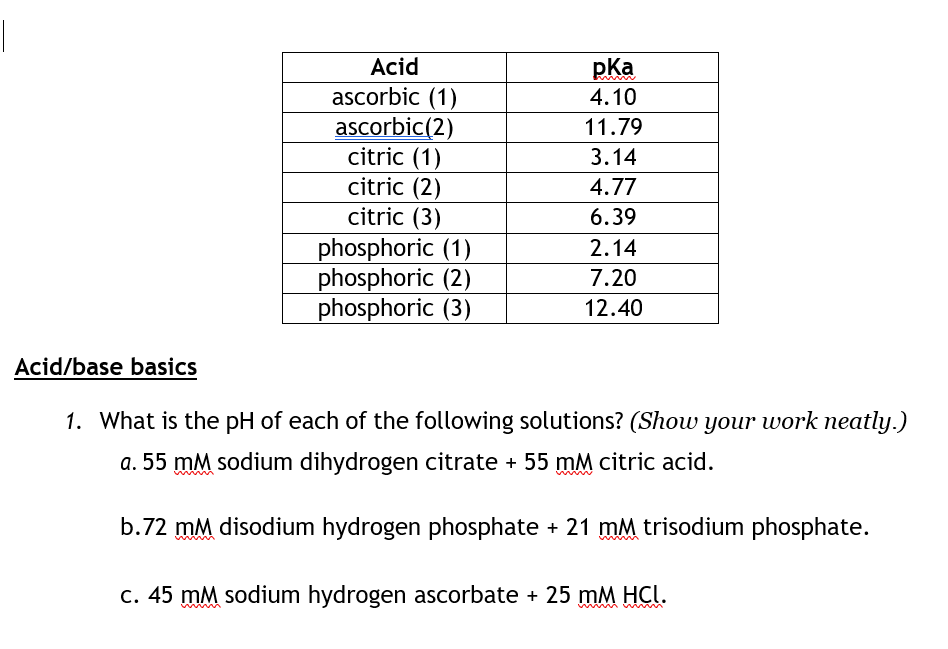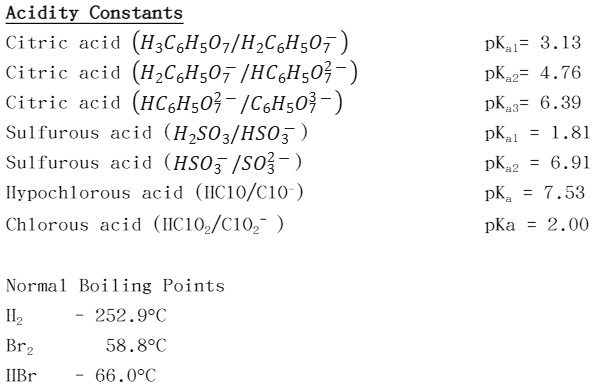
SOLVED: Acidity Constants Citric acid (H3C6HsOn/HzCsHsOv Citric acid (HzC6H50v /HC6H50 Citric acid (HCsHs0 - /CsHs0;- Sulfurous acid (HzSO3/HSO3 Sulfurous acid HSO3 /S0 Ilypochlorous acid (IICIO/C1O Chlorous acid (IICIOz/102 pKal= 3.13 pKaz= 4.76
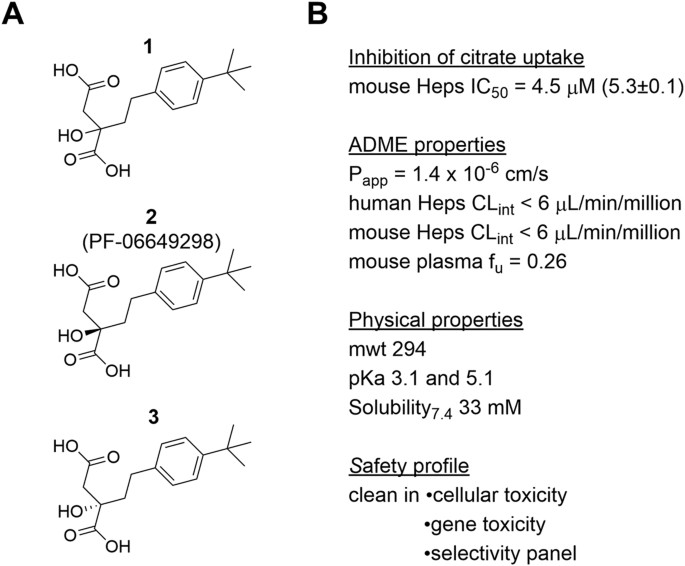
Discovery and characterization of novel inhibitors of the sodium-coupled citrate transporter (NaCT or SLC13A5) | Scientific Reports

Stepwise deprotonation of citric acid (HCitH 3 ) with the respective pK... | Download Scientific Diagram

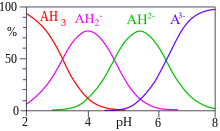
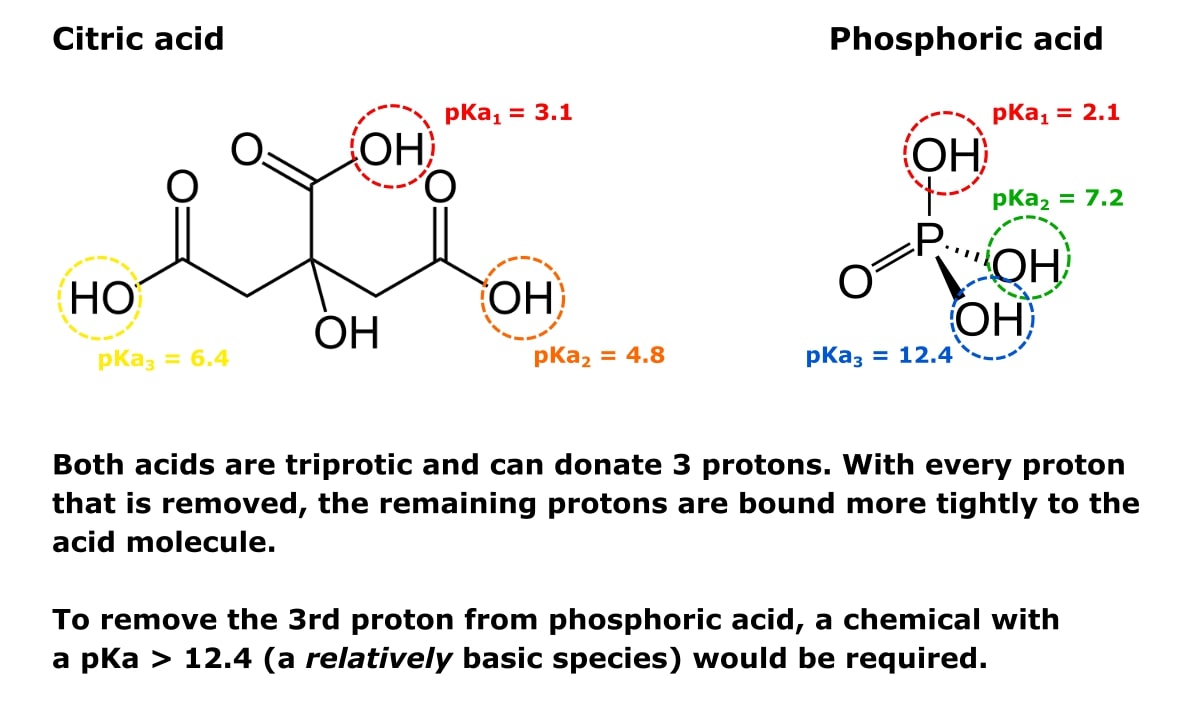
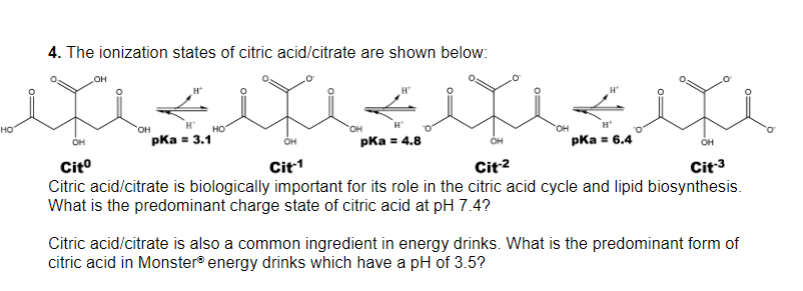
With citric acid considered a triprotic acid, its three corresponding pKa values are 3.1, 4.8, and 6.4. Beyond that, dissociation of the alcohol proton has a pKa value of 14.4 while dissociation

Ionization and Conformational Equilibria of Citric Acid: Delocalized Proton Binding in Solution | The Journal of Physical Chemistry A

Table 1 from Citric acid adsorption on TiO2 nanoparticles in aqueous suspensions at acidic and circumneutral pH: surface coverage, surface speciation, and its impact on nanoparticle-nanoparticle interactions. | Semantic Scholar

Effects of Speciation on the Physical Properties of Frozen Solutions | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology
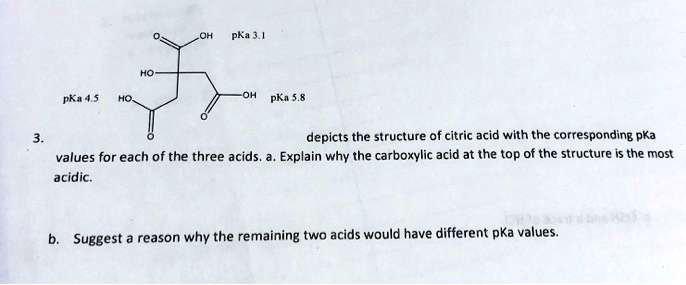
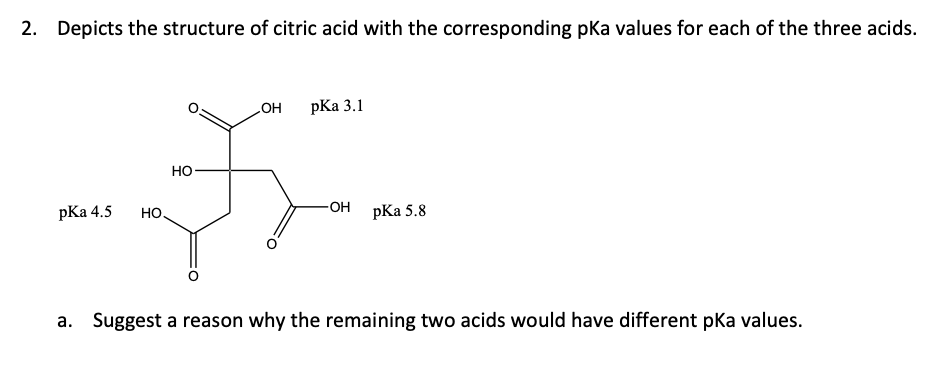
SOLVED: pK44 5 PKa depicts the structure of citric acid with the corresponding pKa values for each of the three acids Explain why the carboxylic acid at the top of the structure
Distribution of citrate ions as a function of pH, computed by Curtipot... | Download Scientific Diagram

SOLVED: Question 19 (1 point) common buffer present in foods is citric acid (pKa 3.11) and its conjugate base: If you need t0 prepare this buffer with PH 3.41,you would need to