
Removal of Sulfate Ions from Calcium Oxide Precipitation Enrichment of a Rare Earth Leaching Liquor by Stirring Washing with Sodium Hydroxide | ACS Omega

How to Balance the Reaction Between Ammonium Nitrate and Calcium Hydroxide NH4NO3 and Ca(OH)2 - YouTube
The remaining gas is mainly nitrogen containing some impurities of rare gases which cannot be removed by any chemical means as

Write the chemical formula of the following. (a) Magnesium chloride (b) Calcium oxide (c) Copper nitrate (d) Aluminium chloride (e) Calcium carbonate

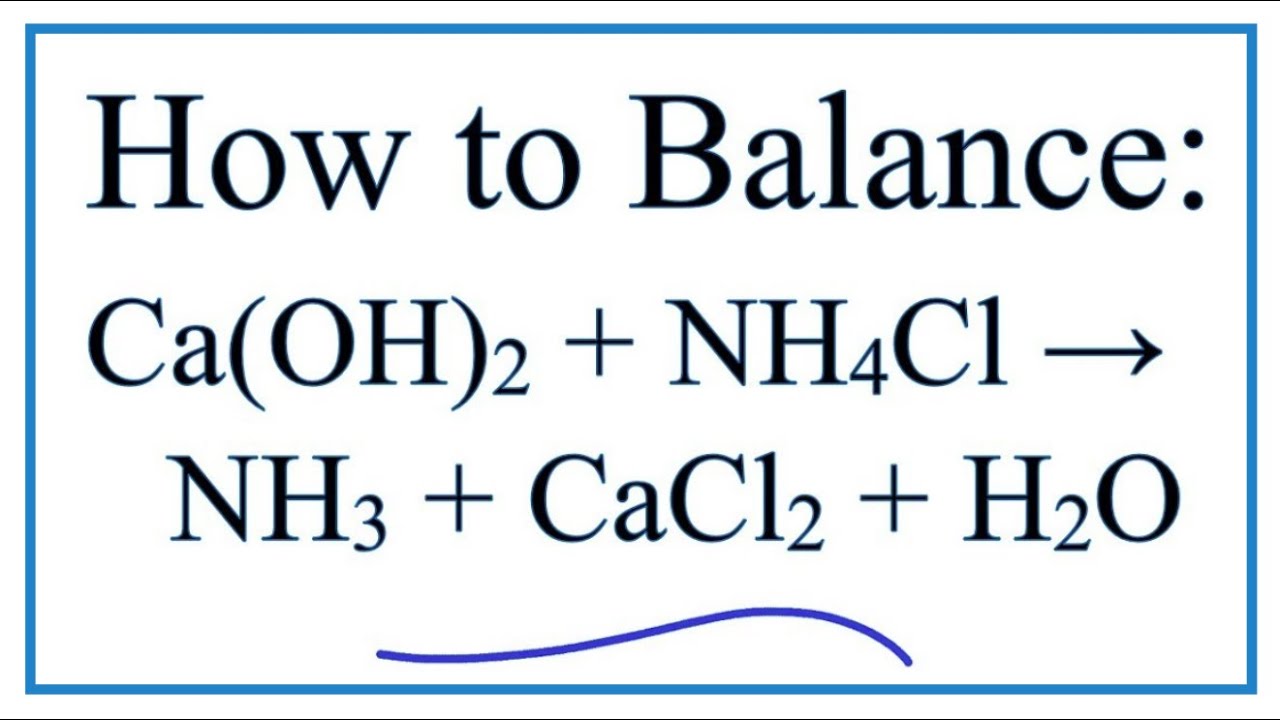
Calcium hydroxide and ammonium chloride react to give ammonia as per equation: Ca(OH)_(2) + 2 NH... - YouTube
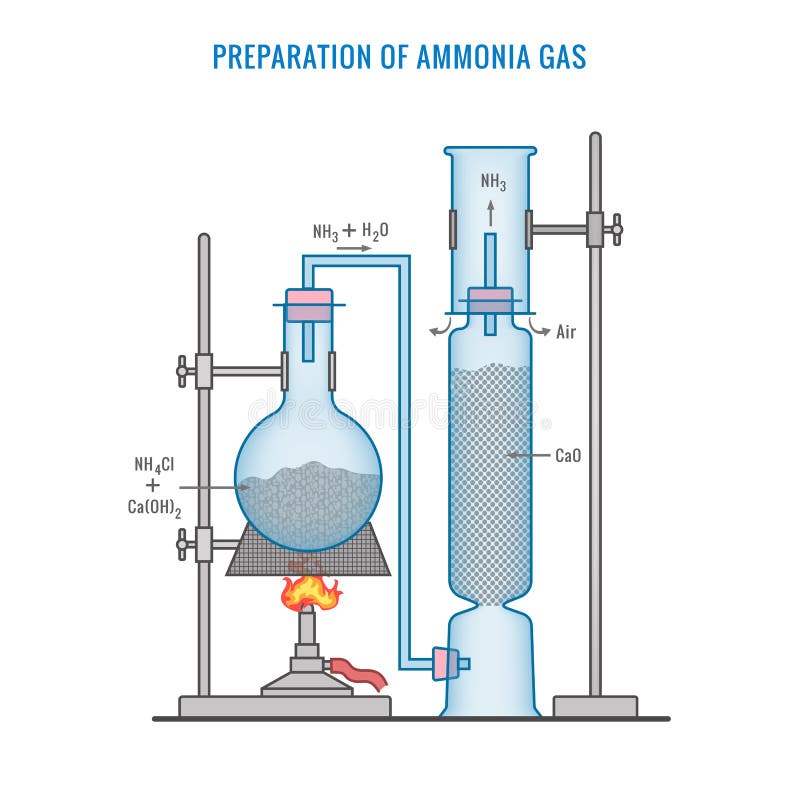
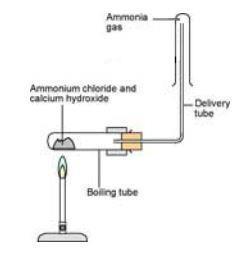
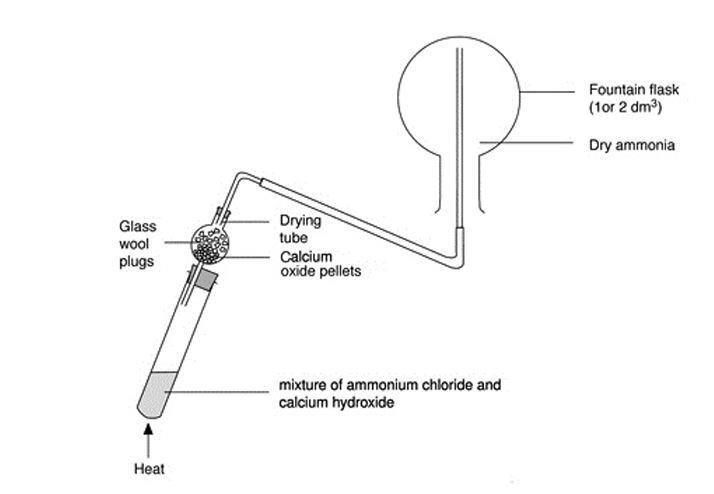
Preparation of Ammonia Gas in Laboratory with the Help of Ammonium Chloride and Calcium Oxide Stock Vector - Illustration of white, formula: 220304379

List the characteristics of cork. How are they formed? Mention their role.a) Write the formula of (i) Magnesium hydroxide (ii) Hydrogen sulphide (iii) Potassium chloride (iv) Calcium oxide (v) Barium chloride (vi)
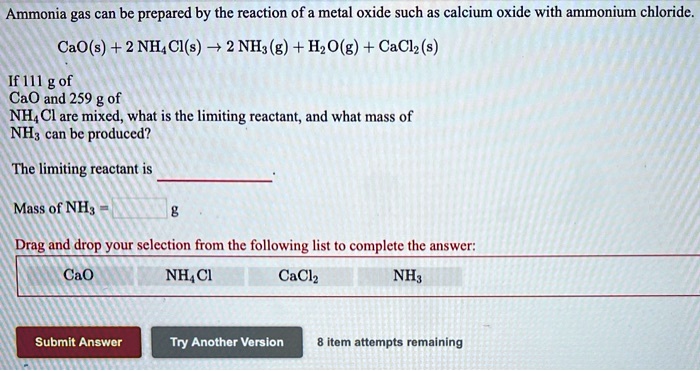
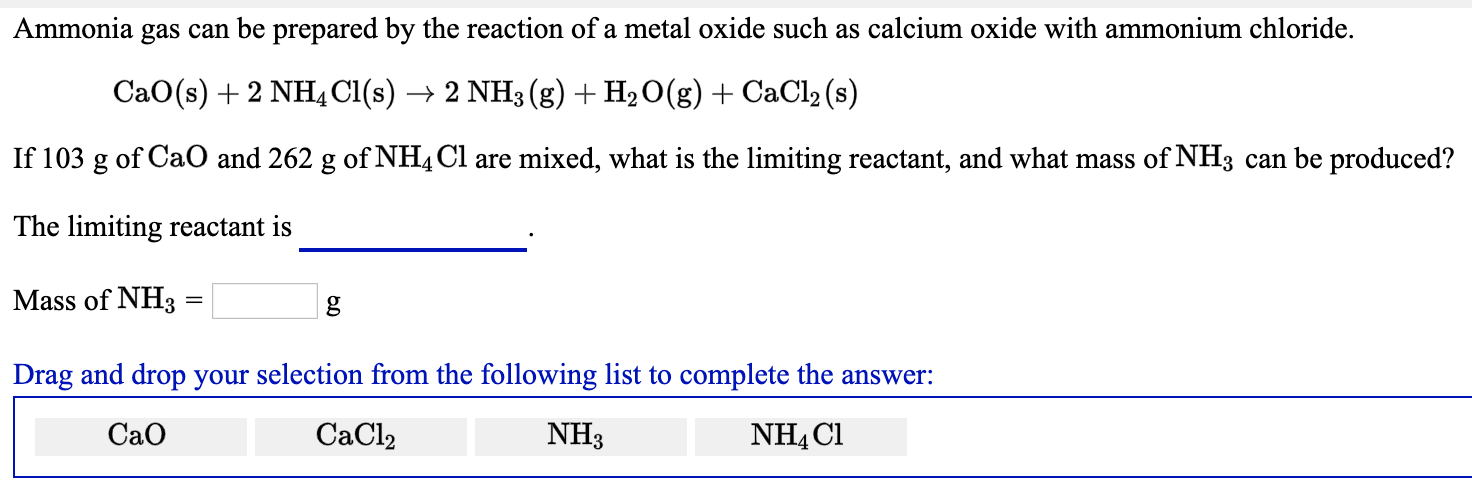
SOLVED: Ammonia gas can be prepared by the reaction of metal oxide such as calcium oxide with ammonium chloride. CaO(s) + 2 NH,CI(s) 2 NHs(g) + Hz(g) + CaClz (s) If I1

Nitrogen has a triple bond which is very strong. :N:::N: Only at very high temperatures will it react with oxygen. This occurs in the combustion. - ppt download
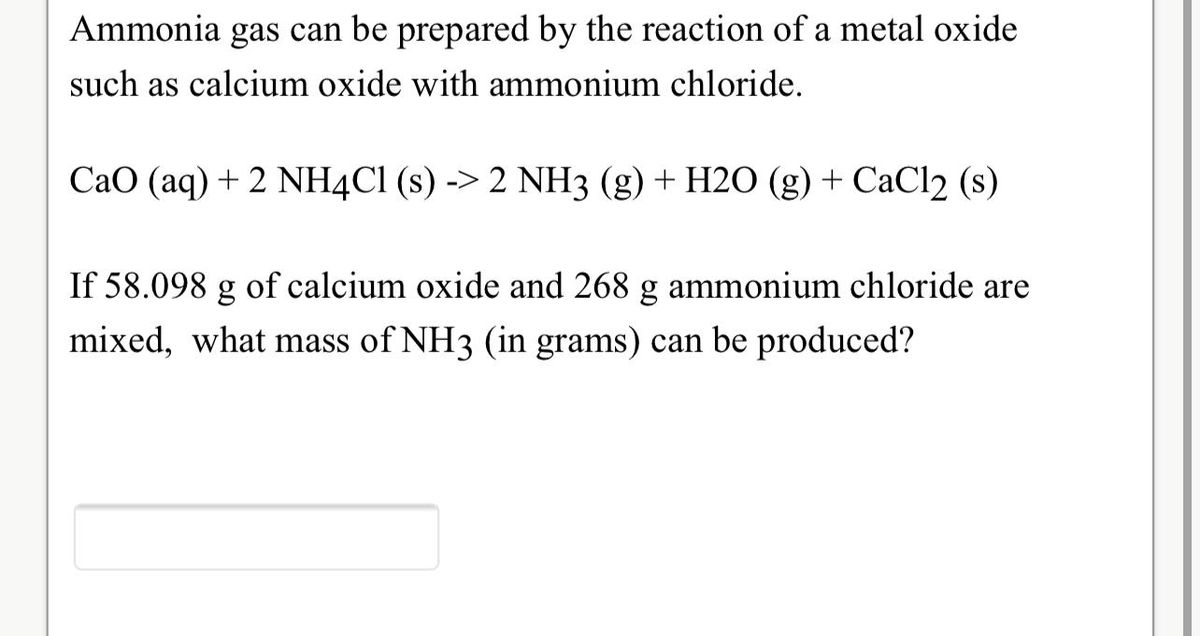
Obtained when 32.6g. of ammonium chloride reacts with calcium hydroxide during the laboratory. - Sarthaks eConnect | Largest Online Education Community
![Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2021/10/qa-aq-ammonia.jpg?w=986)
Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition










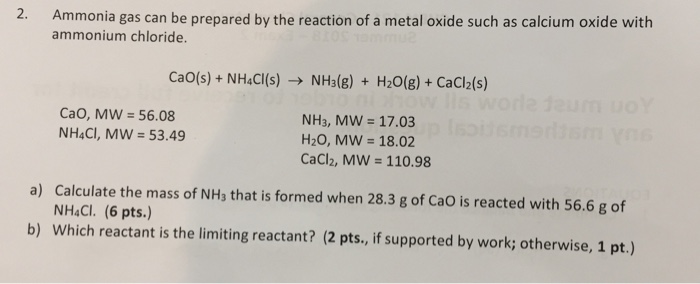
.png)