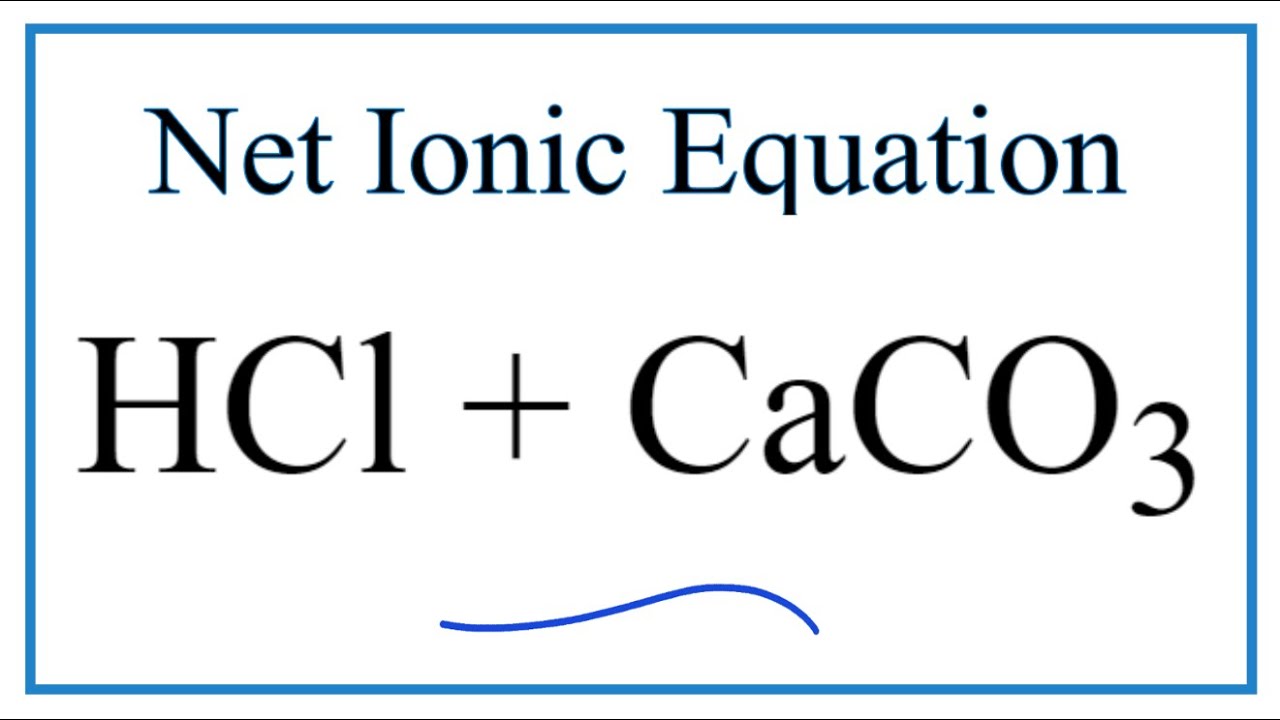Question Video: Calculating the Average Rate of Reaction of Hydrochloric Acid with Calcium Carbonate | Nagwa

a) Substitute formulae for names and balance the following equation: Calcium carbonate reacts - YouTube

Concentrated hydrochloric acid reats more vigorously with calcium carbonate than dilute hydrochoric acid.

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa

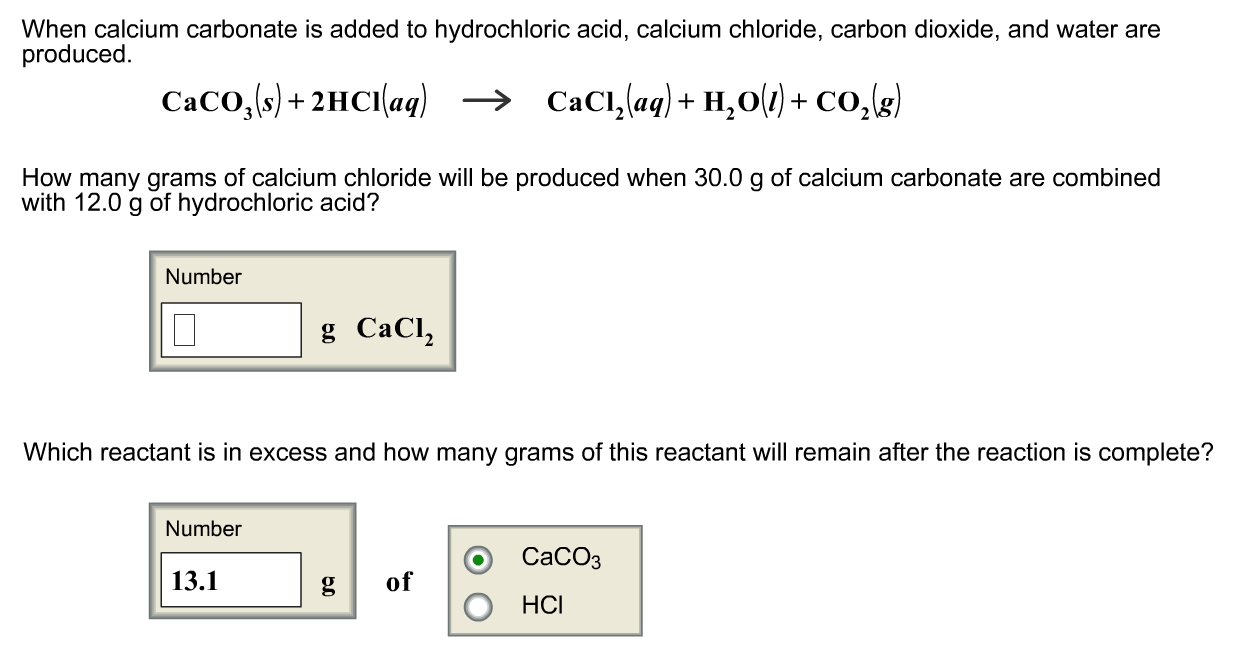
CaCO3 + 2HCl → CaCl2 + H2O + CO2 The mass of calcium chloride formed when 2.5 g of calcium carbonate is dissolved in excess of hydrochloric acid is:
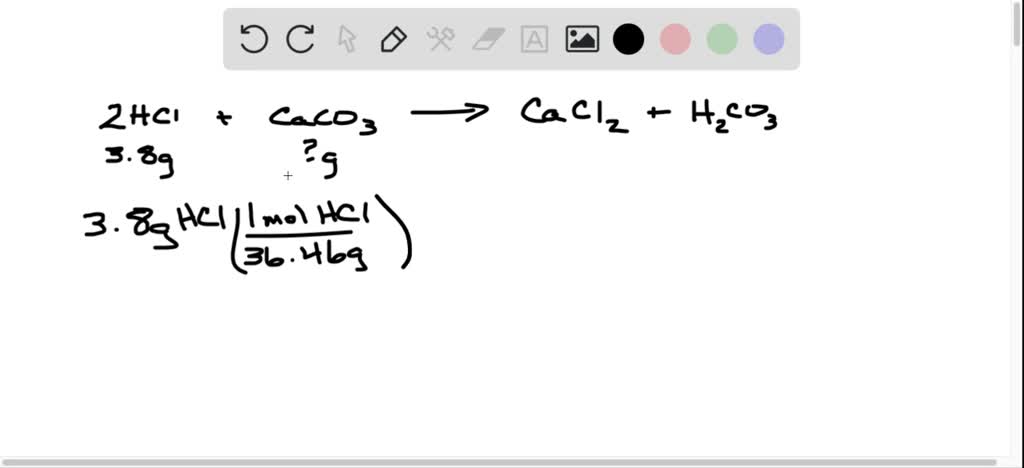
SOLVED: Toilet bowl cleaners often contain hydrochloric acid, which dissolves the calcium carbonate deposits that accumulate within a toilet bowl. What mass of calcium carbonate (in grams) can 3.8 g of HCl

calcium carbonate reacts with dilute hydrochloric acid to produce carbon dioxide Stock Photo - Alamy

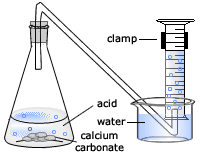
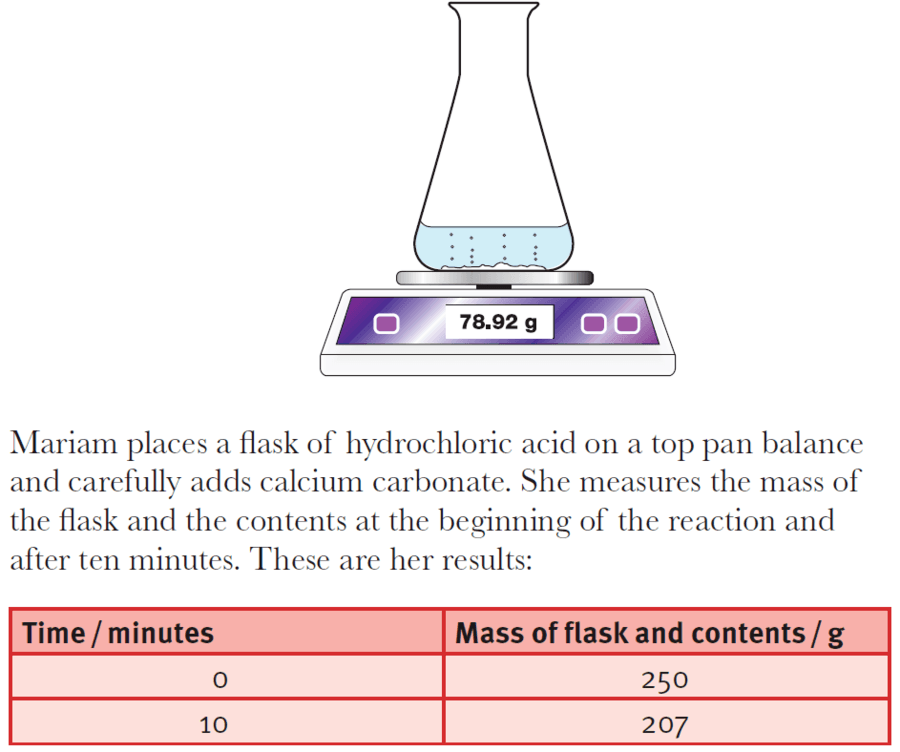
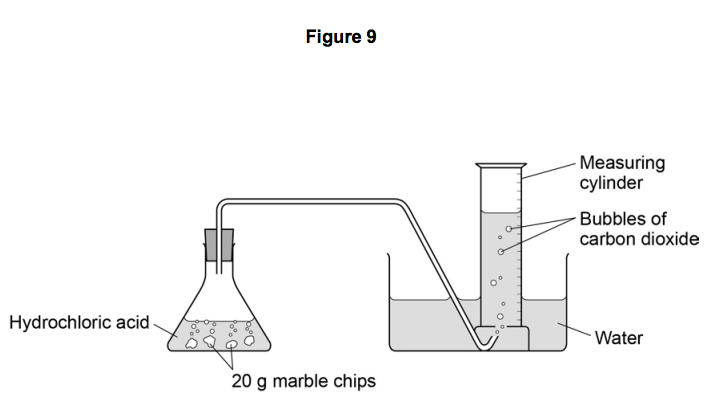
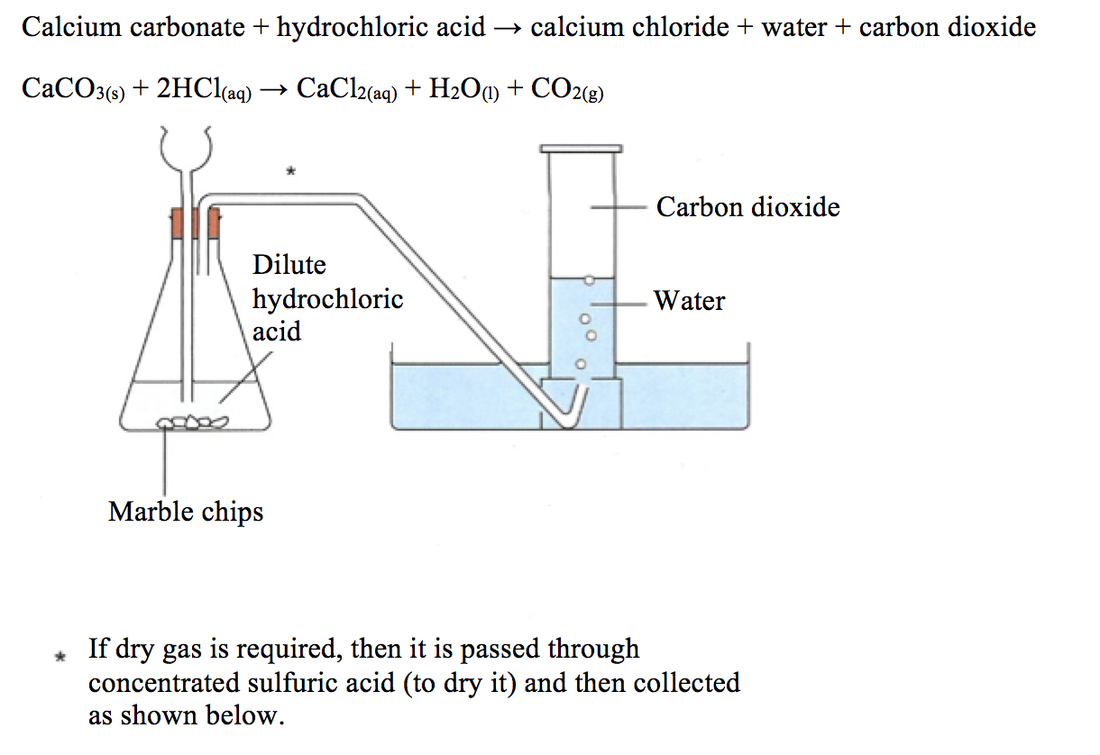
Describe an experiment to study the speed of reaction between calcium carbonate and dilute hydrochloric acid, by measuring the loss in mass of reaction system over time. - Study notes, tips, worksheets,