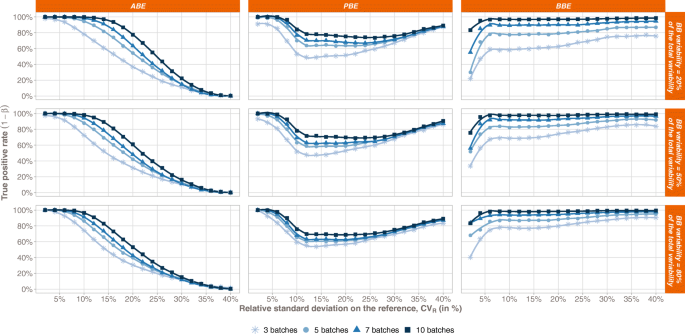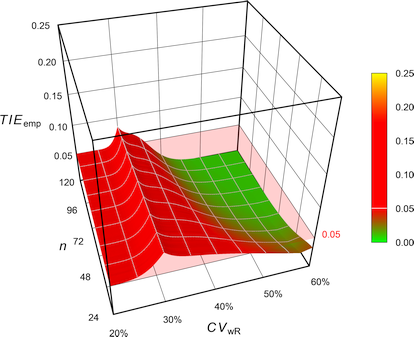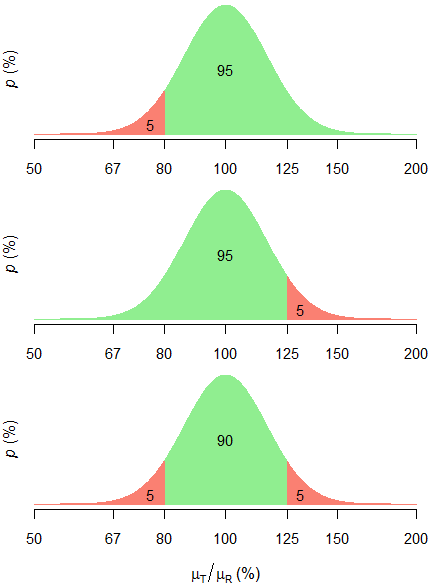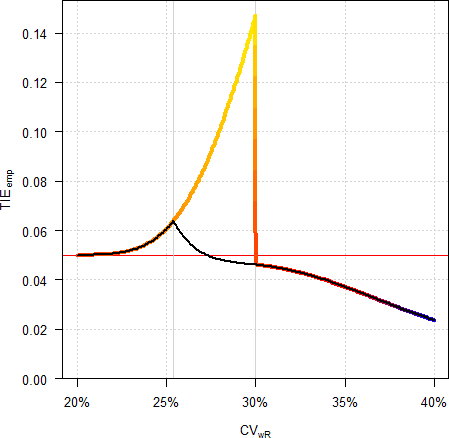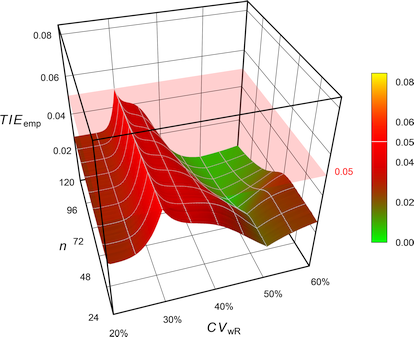
Pharmaceuticals | Free Full-Text | Model-Based Approach for Designing an Efficient Bioequivalence Study for Highly Variable Drugs

Sample size determination in bioequivalence studies using statistical assurance - Ring - 2019 - British Journal of Clinical Pharmacology - Wiley Online Library
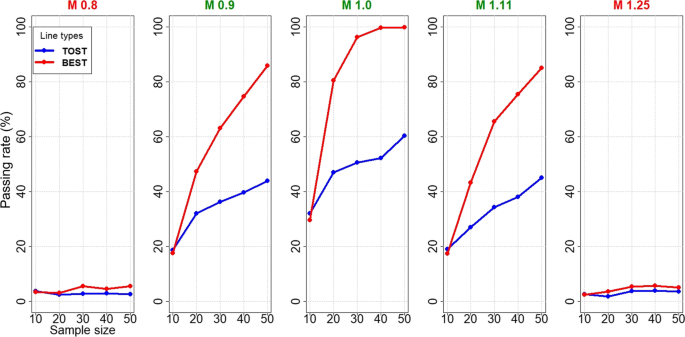
Comparing a Bayesian Approach (BEST) with the Two One-Sided t-Tests (TOSTs) for Bioequivalence Studies | SpringerLink

NASAL SPRAY BIOEQUIVALENCE - Between-Batch Bioequivalence (BBE): An Alternative Statistical Method to Assess In Vitro Bioequivalence of Nasal Product

Percent of studies passing bioequivalence (BE) (power curves); average... | Download Scientific Diagram

A visual representation of some possible results of the statistical... | Download Scientific Diagram

Pharmaceuticals | Free Full-Text | Model-Based Approach for Designing an Efficient Bioequivalence Study for Highly Variable Drugs
Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial | PLOS Medicine

The 90% confidence interval for average bioequivalence measures (Cmax... | Download Scientific Diagram

Implementation of a reference-scaled average bioequivalence approach for highly variable generic drug products of agomelatine in Chinese subjects - ScienceDirect

Pharmaceutics | Free Full-Text | Model-Based Equivalent Dose Optimization to Develop New Donepezil Patch Formulation
Novel Model-Integrated Design for Bioequivalence Studies of LAI Products A Complete Framework with the MonolixSuite
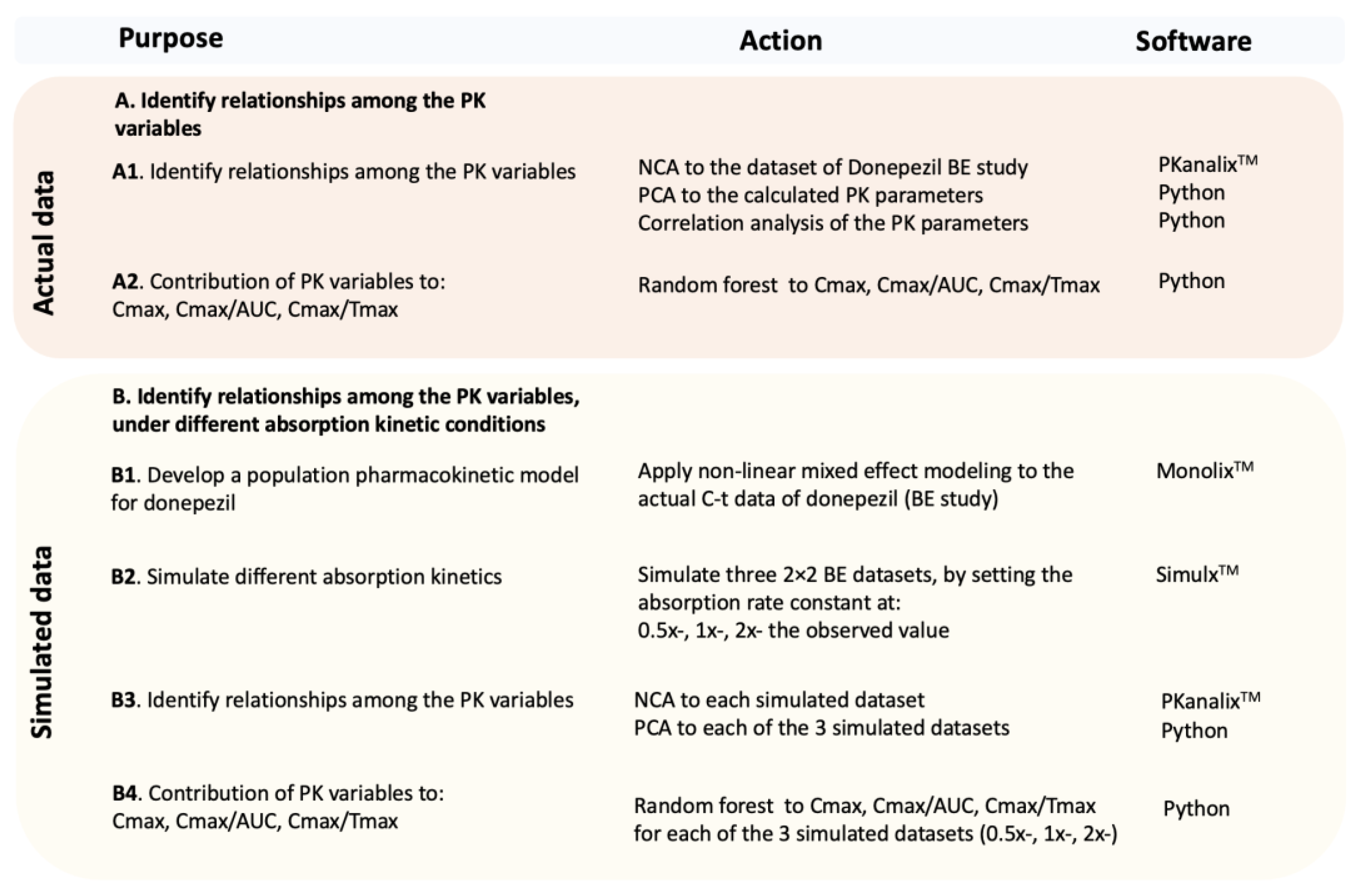
Applied Sciences | Free Full-Text | Machine Learning in Bioequivalence: Towards Identifying an Appropriate Measure of Absorption Rate
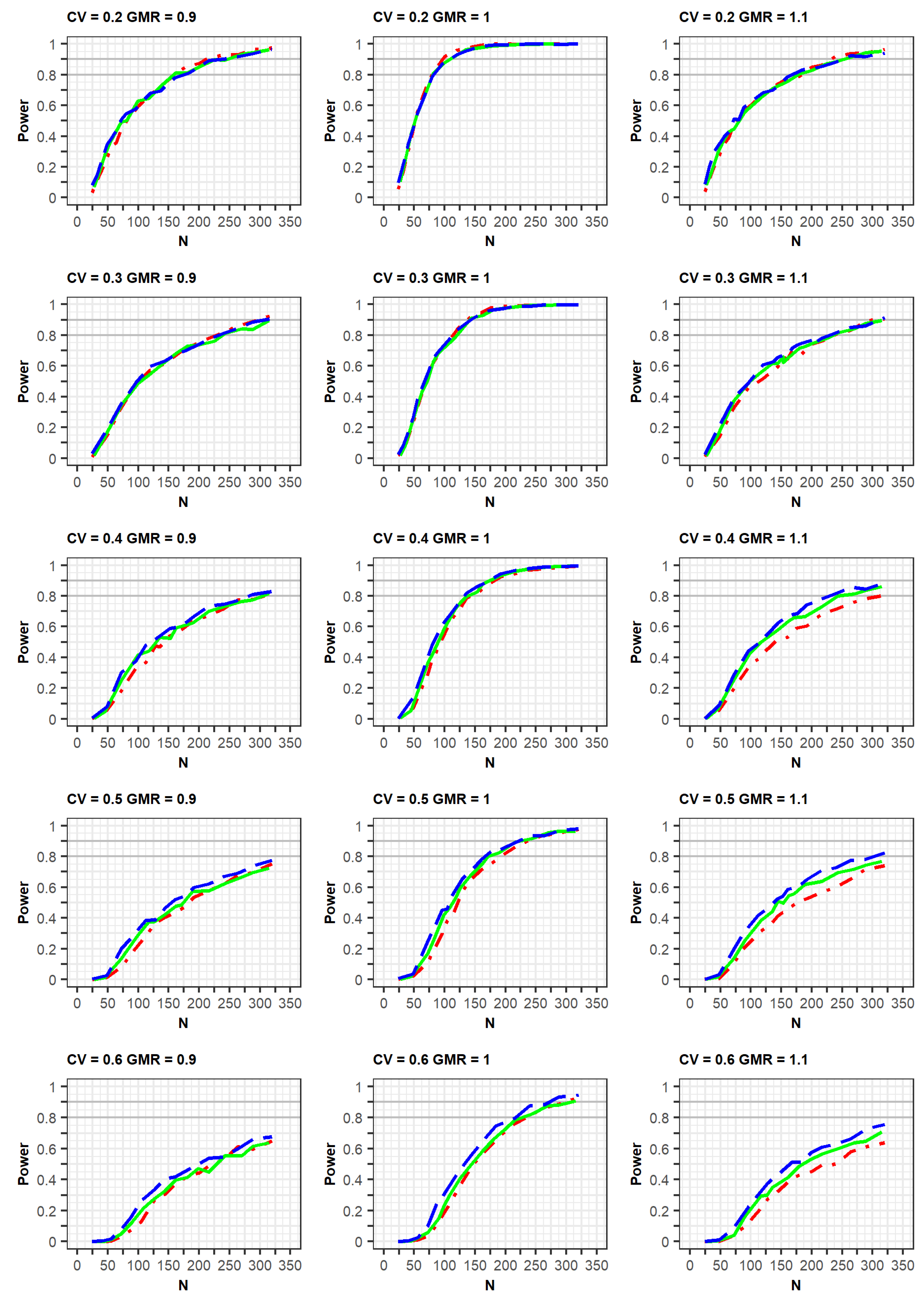
Power curves for the determination of bioequivalence. The conditions... | Download Scientific Diagram

Methods to control the empirical type I error rate in average bioequivalence tests for highly variable drugs - Yuhao Deng, Xiao-Hua Zhou, 2020

Pharmaceuticals | Free Full-Text | Model-Based Approach for Designing an Efficient Bioequivalence Study for Highly Variable Drugs