
Bi2(C2O4)3·7H2O and Bi(C2O4)OH Oxalates Thermal Decomposition Revisited. Formation of Nanoparticles with a Lower Melting Point than Bulk Bismuth | Inorganic Chemistry

Multiple dielectric relaxations and superior sonocatalysis of bismuth iron niobate pyrochlores via high-level Co-doping - ScienceDirect
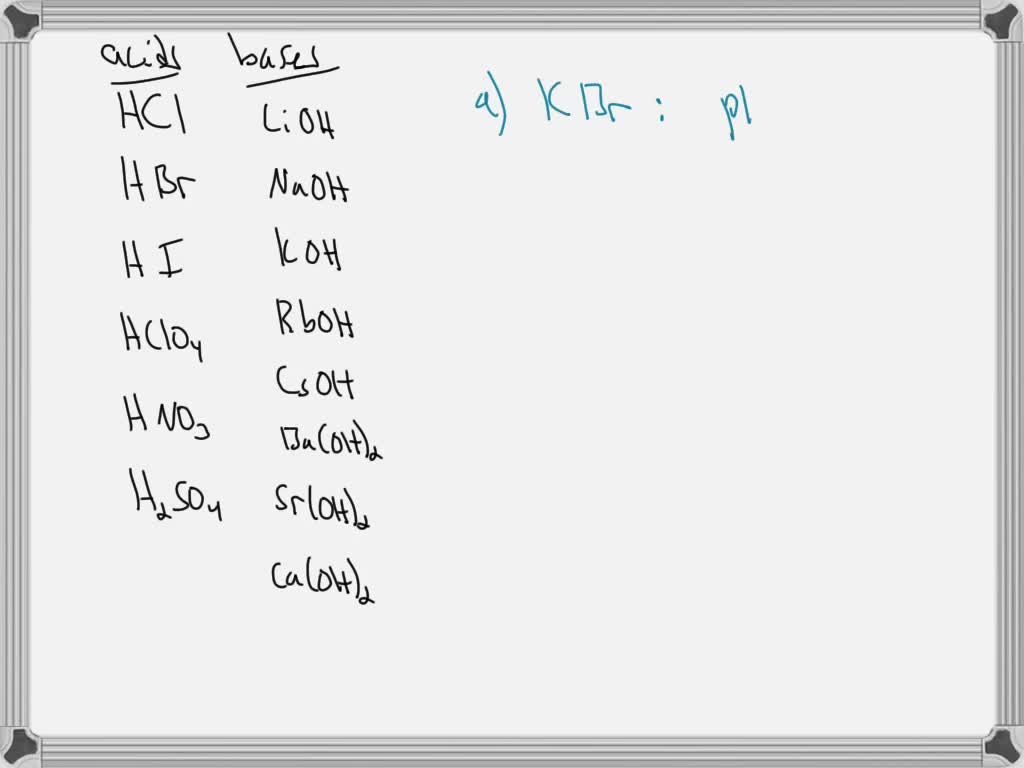
SOLVED: 7 Determine whether aqueous solutions of the following salts have pH equal to, greater than, or less than 7; ifpH > 7 or pH < 7, write a chemical equation to

Magnesium is precipitated from its salt solution as only magnesium ammonium phosphate by adding disodium hydrogen phosphate solution in absence of ammonium chloride and aqueous ammonia.


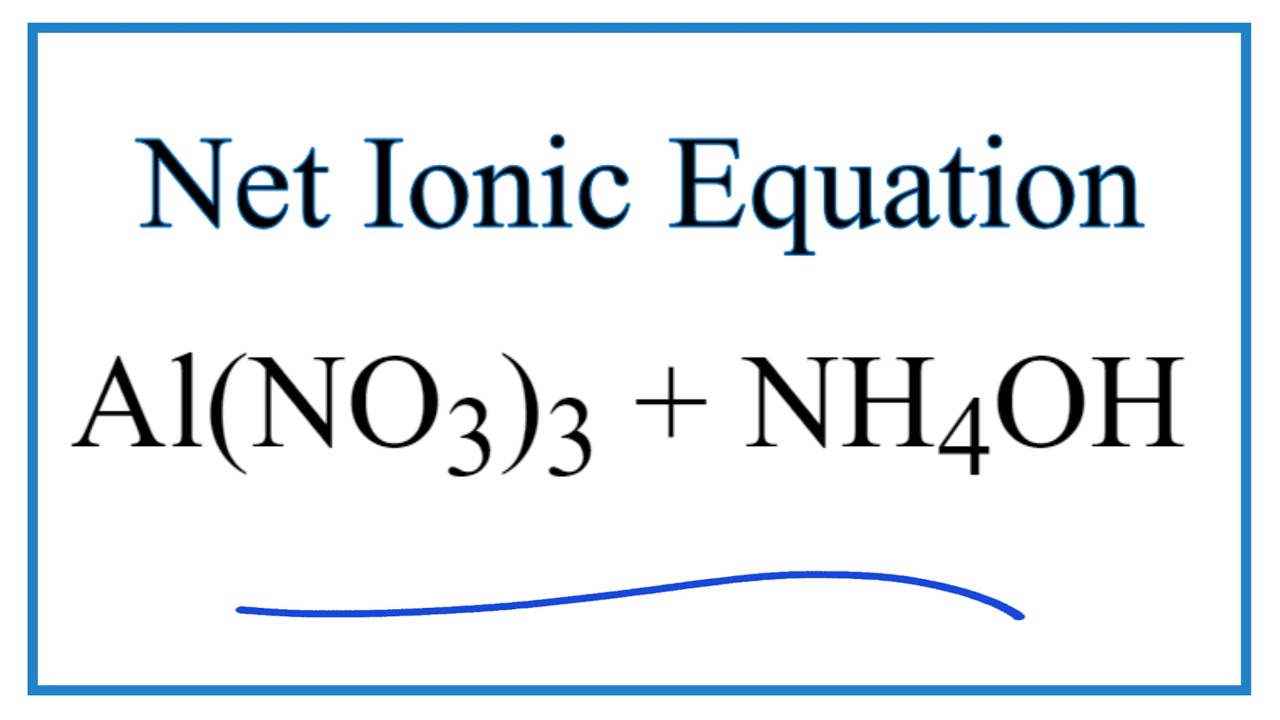

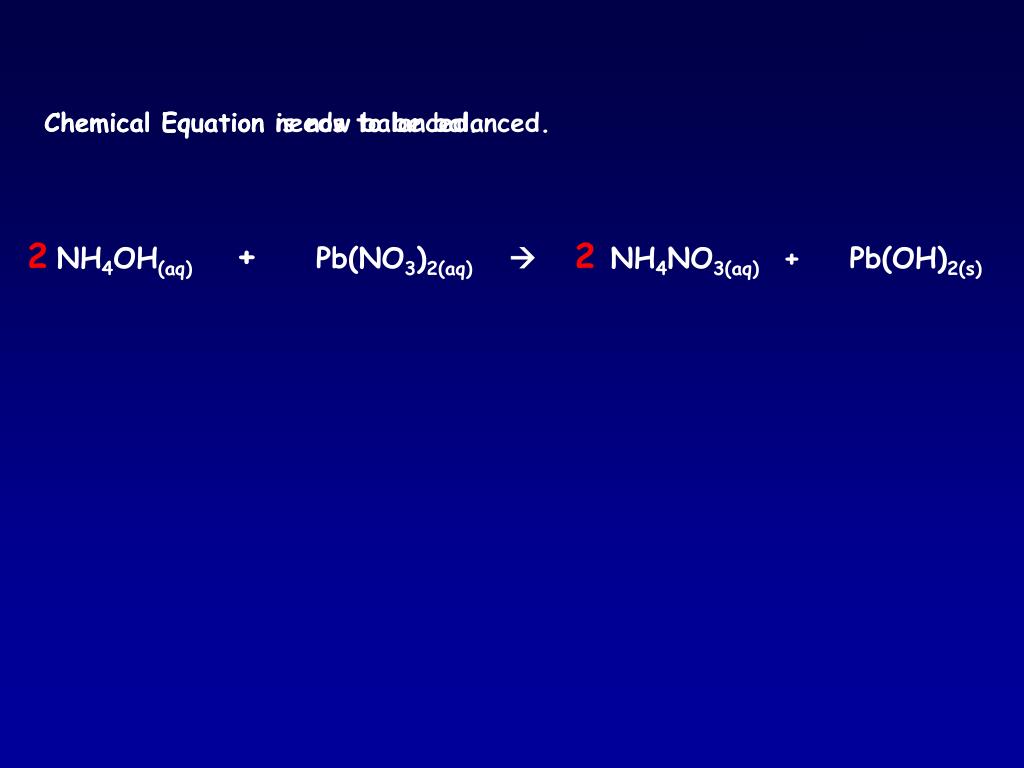
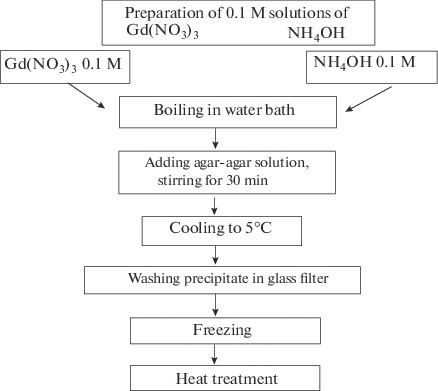





3%20+%20NaOH%20=%20Bi(OH)3%20+%20NaNO3.svg)