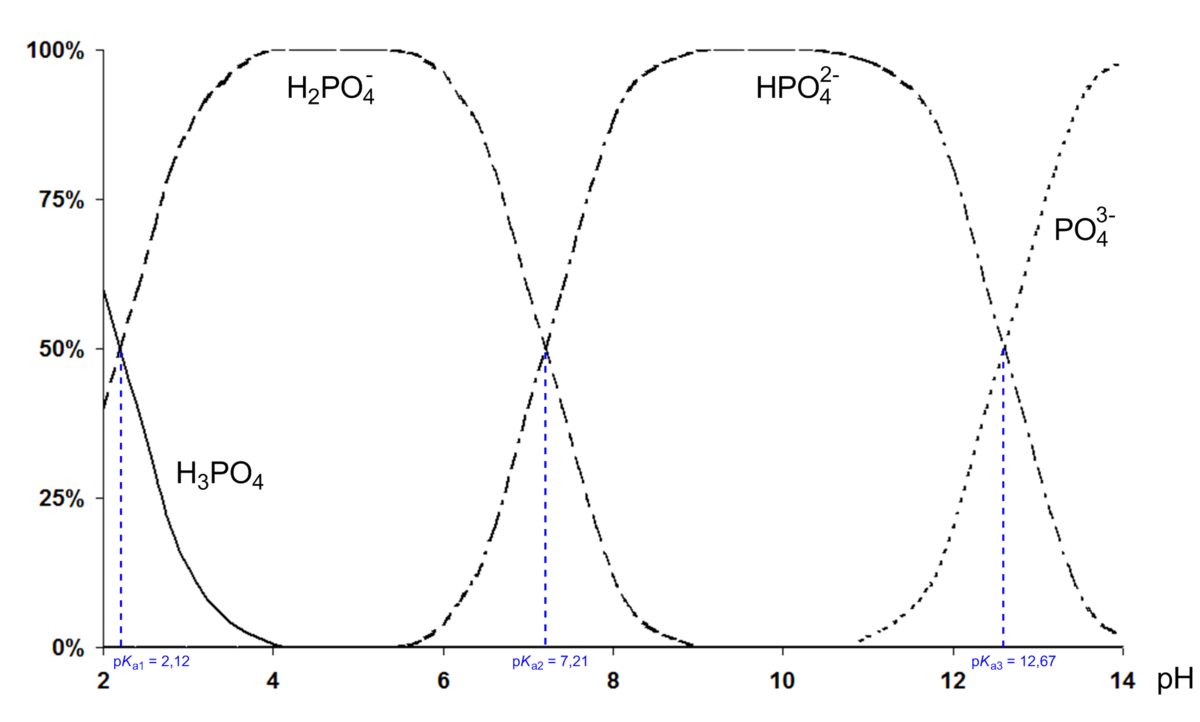
equilibrium - How to calculate the dissociation constant of a weak acid from the titration with a strong base? - Chemistry Stack Exchange

SOLVED:Acetic acid dissociates in solution according to the following equation: CH3COOH⇌CH3COO^-+H^+ If sodium acetate is added to a solution of acetic acid in excess water, which of the following effects would be

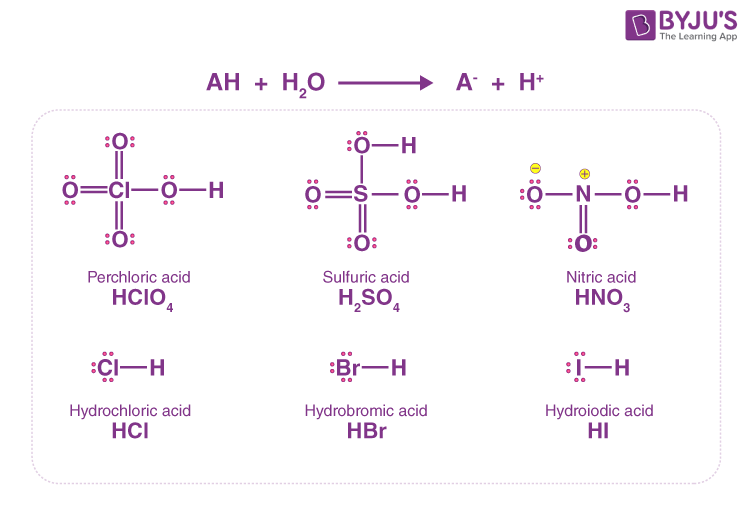
Dissociation Constants of Perchloric and Sulfuric Acids in Aqueous Solution | The Journal of Physical Chemistry B
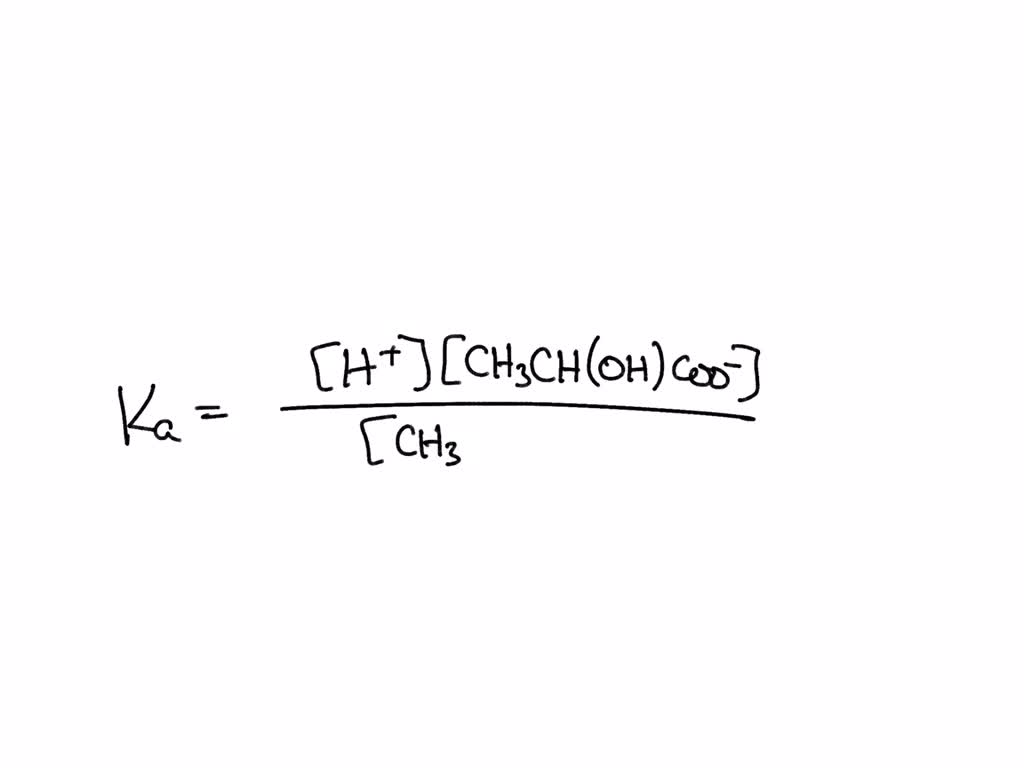
SOLVED: The following equation shows dissociation of lactic acid. CH3CH(OH)CO2H—> H+ + CH3CH(OH)COO-Write and equation to define the dissociation constant (Ka) for the acid.

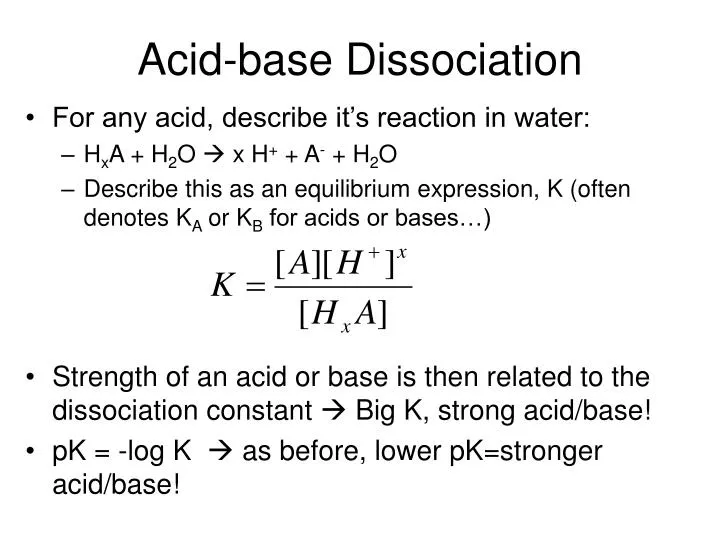
ACIDS AND BASES Dissociation Constants. weaker the acid, the stronger its conjugate base stronger the acid, the weaker its conjugate base. - ppt download









:max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)






![PDF] 238 Amino Acid Dissociation Constants | Semantic Scholar PDF] 238 Amino Acid Dissociation Constants | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/21cc4c546f827dd86621a20daf93772375732463/11-TableI-1.png)