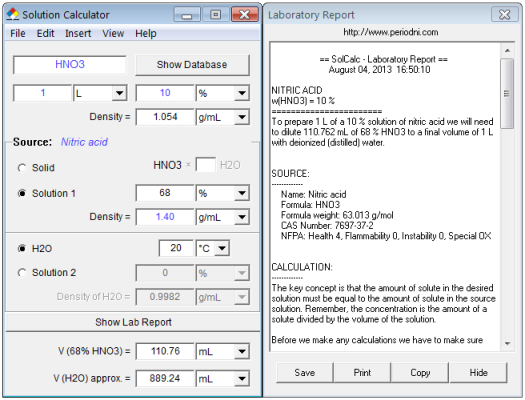
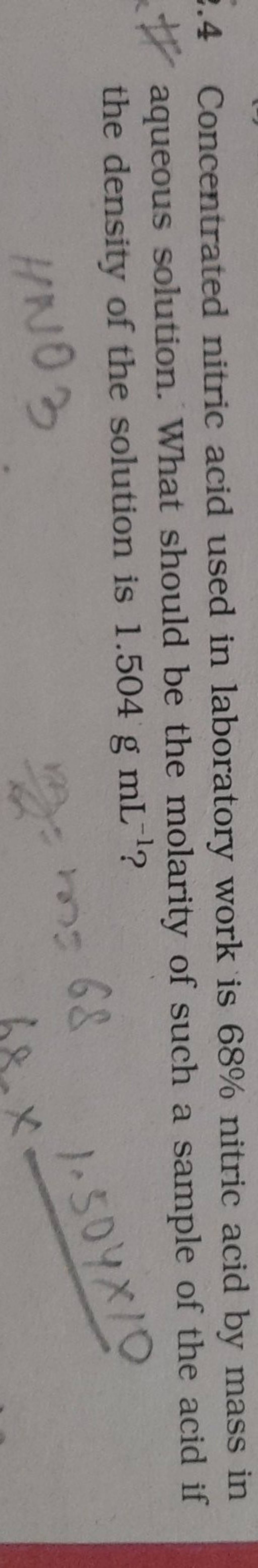How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0 M HNO3 ? The concentrated acid is 70

Graph of molarity of nitric acid versus uranium extraction efficiency... | Download Scientific Diagram

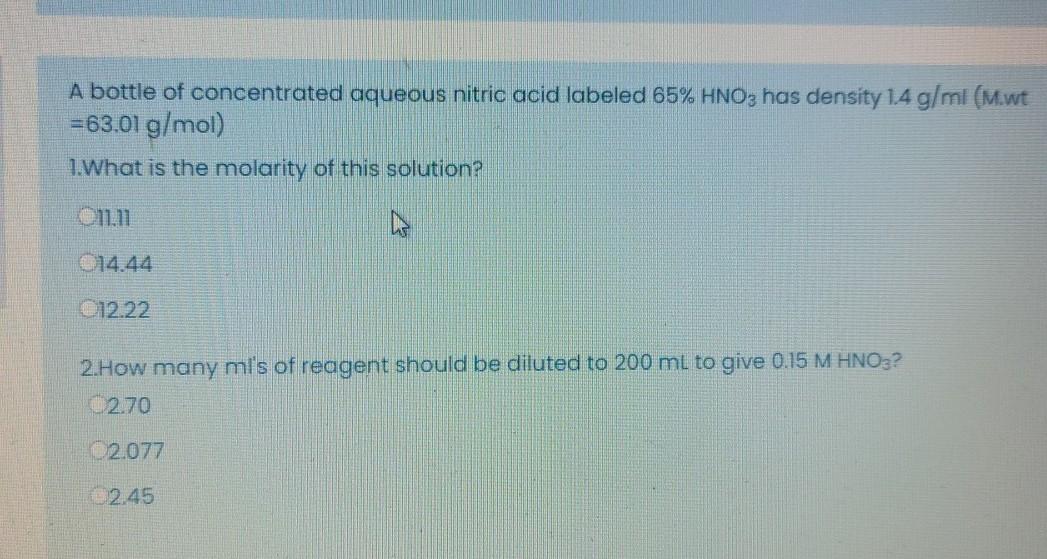
SOLVED: A bottle of concentrated aqueous nitric acid labeled 65% HNOz has density 1.4 g/ml (Mwt =63.01 g/mol) 1What is the molarity of this solution? ILII 214.44 1222 2How many mls of

Calculate the concentration of nitric acid in moles per litre in a sample which has a density `1... - YouTube

The student will: be able to explain the experimental technique of titration. math calculate the molarity or volume of an unknown solution using the titration. - ppt download

SOLVED:A 30.00 % -by-mass solution of nitric acid, HNO3, in water has a density of 1.18 g / cm^3 at 20^∘ C. What is the molarity of HNO3 in this solution?